Leveraging Engineered Pseudomonas putida Minicells for Bioconversion of Organic Acids into Short-Chain Methyl Ketones
- PMID: 39748701
- PMCID: PMC11744930
- DOI: 10.1021/acssynbio.4c00700
Leveraging Engineered Pseudomonas putida Minicells for Bioconversion of Organic Acids into Short-Chain Methyl Ketones
Abstract
Methyl ketones, key building blocks widely used in diverse industrial applications, largely depend on oil-derived chemical methods for their production. Here, we investigated biobased production alternatives for short-chain ketones, adapting the solvent-tolerant soil bacterium Pseudomonas putida as a host for ketone biosynthesis either by whole-cell biocatalysis or using engineered minicells, chromosome-free bacterial vesicles. Organic acids (acetate, propanoate and butanoate) were selected as the main carbon substrate to drive the biosynthesis of acetone, butanone and 2-pentanone. Pathway optimization identified efficient enzyme variants from Clostridium acetobutylicum and Escherichia coli, tested with both constitutive and inducible expression of the cognate genes. By implementing these optimized pathways in P. putida minicells, which can be prepared through a simple three-step purification protocol, the feedstock was converted into the target short-chain methyl ketones. These results highlight the value of combining morphology and pathway engineering of noncanonical bacterial hosts to establish alternative bioprocesses for toxic chemicals that are difficult to produce by conventional approaches.
Keywords: 2-pentanone; Pseudomonas putida; acetone; butanone; ketones; metabolic engineering; minicells; synthetic biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
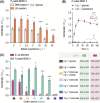
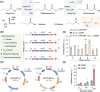

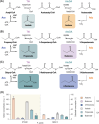
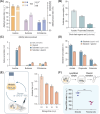
References
-
- Grütering C.; Honecker C.; Hofmeister M.; Neumann M.; Raßpe-Lange L.; Du M.; Lehrheuer B.; von Campenhausen M.; Schuster F.; Surger M.; et al. Methyl ketones: a comprehensive study of a novel biofuel. Sust. Energy Fuels 2024, 8, 2059–2072. 10.1039/D4SE00035H. - DOI
-
- Dong J.; Chen Y.; Benites V. T.; Baidoo E. E. K.; Petzold C. J.; Beller H. R.; Eudes A.; Scheller H. V.; Adams P. D.; Mukhopadhyay A.; et al. Methyl ketone production by Pseudomonas putida is enhanced by plant-derived amino acids. Biotechnol. Bioeng. 2019, 116, 1909–1922. 10.1002/bit.26995. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

