Identification and Management of CKD-Associated Pruritus: Current Insights
- PMID: 39748827
- PMCID: PMC11693948
- DOI: 10.2147/IJNRD.S499798
Identification and Management of CKD-Associated Pruritus: Current Insights
Abstract
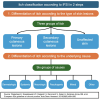
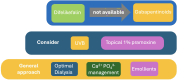
Chronic kidney disease-associated pruritus (CKD-aP) is a frequent and distressing problem for individuals with chronic kidney disease (CKD) and end-stage renal disease. It affects around 20% of those with CKD and 40% of those with end-stage renal disease. Despite its clear association with poorer psychosocial and medical outcomes, it is often underreported by patients and frequently remains unnoticed by healthcare providers. This is likely due to uncertainty regarding its diagnosis and treatment. Most commonly, CKD-aP could be screened with questionnaires like the KDQoL-36 and WI-NRS, chosen for their simplicity and ease of use. Prior treatment studies of CKD-aP were mostly limited by noncontrolled design and small sample size. First CKD-aP medication - difelikefalin a powerful, new therapeutic option was approved by Federal Drug Administration (FDA) in 2021 and European Medicines Agency (EMA) in 2022. Recent expert opinions, clinical trials and metanalysis identified difelikefalin and gabapentinoids as medications of choice in treatment of CKD-aP. All these findings improved current understanding and management of this condition.
Keywords: chronic kidney disease; difelikefalin; gabapentin; itch; pregabalin; pruritus.
© 2024 Skrzypczak et al.
Conflict of interest statement
Jacek C Szepietowski has served as an advisor for AbbVie, LEO Pharma, Menlo Therapeutics, Novartis, Pierre Fabre, Sienna Biopharmaceuticals, and Trevi; has received speaker honoraria from AbbVie, Eli Lilly, Janssen, LEO Pharma, Novartis, Sanofi-Genzyme, Sun Pharma, and Berlin-Chemie Mennarini; has served as an investigator; and has received funding from AbbVie, Amgen, Galapagos, Holm, Incyte Corporation, InflaRX, Janssen, Menlo Therapeutics, Merck, Boehringer Ingelheim, Novartis, Almirall, Pfizer, Regeneron, Trevi, UCB, and personal fees from Vifor, during the conduct of the study. Other authors report no conflicts of interest in this work.
Figures
Similar articles
-
CKD-Associated Pruritus: New Insights Into Diagnosis, Pathogenesis, and Management.Kidney Int Rep. 2020 May 8;5(9):1387-1402. doi: 10.1016/j.ekir.2020.04.027. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954065 Free PMC article. Review.
-
Cost-utility analysis of difelikefalin for the treatment of moderate to severe Chronic Kidney Disease associated-Pruritus (CKD-aP) in adult patients receiving haemodialysis in Spain.J Med Econ. 2025 Dec;28(1):835-847. doi: 10.1080/13696998.2025.2501874. Epub 2025 Jun 4. J Med Econ. 2025. PMID: 40411773
-
Difelikefalin improves itch-related sleep disruption in patients undergoing haemodialysis.Nephrol Dial Transplant. 2024 Jun 28;39(7):1125-1137. doi: 10.1093/ndt/gfad245. Nephrol Dial Transplant. 2024. PMID: 37968132 Free PMC article. Clinical Trial.
-
Efficacy of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis of KALM-1 and KALM-2 Phase 3 Studies.Kidney Med. 2022 Jun 28;4(8):100512. doi: 10.1016/j.xkme.2022.100512. eCollection 2022 Aug. Kidney Med. 2022. PMID: 36016762 Free PMC article.
-
An evaluation of difelikefalin as a treatment option for moderate-to-severe pruritus in end stage renal disease.Expert Opin Pharmacother. 2021 Apr;22(5):549-555. doi: 10.1080/14656566.2020.1849142. Epub 2020 Dec 14. Expert Opin Pharmacother. 2021. PMID: 33190563 Review.
References
-
- Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):i–ii+. - PubMed
-
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Renal Fibrosis. 2019;3–15. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials