Discovery and clinical translation of ceperognastat, an O-GlcNAcase (OGA) inhibitor, for the treatment of Alzheimer's disease
- PMID: 39748851
- PMCID: PMC11694536
- DOI: 10.1002/trc2.70020
Discovery and clinical translation of ceperognastat, an O-GlcNAcase (OGA) inhibitor, for the treatment of Alzheimer's disease
Abstract
Introduction: The aggregation and spread of hyperphosphorylated, pathological tau in the human brain is hypothesized to play a key role in Alzheimer's disease (AD) as well as other neurogenerative tauopathies. O-GlcNAcylation, an important post-translational modification of tau and many other proteins, is significantly decreased in brain tissue of AD patients relative to healthy controls. Increased tau O-GlcNAcylation has been shown to reduce tau pathology in mouse in vivo tauopathy models. O-GlcNAcase (OGA) catalyzes the removal of O-GlcNAc from tau thereby driving interest in OGA inhibition as a potential therapeutic approach to reduce tau pathology and slow the progression of AD.
Methods: A multidisciplinary approach was used to identify ceperognastat (LY3372689) as a potent OGA inhibitor, including an extensive discovery effort with synthetic chemistry, structure-based drug design, and in vivo OGA enzyme occupancy studies. Preclinical studies assessed the target engagement, inhibition of OGA enzyme activity, OGA enzyme occupancy, and changes in tau O-GlcNAc. Four clinical Phase 1 studies of ceperognastat in healthy participants were performed to assess clinical safety and tolerability, pharmacokinetics (PK), and enzyme occupancy.
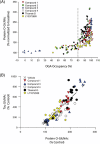
Results: Ceperognastat is a potent, central nervous system (CNS)-penetrant, low-dose inhibitor of OGA, which can achieve > 95% OGA enzyme occupancy in animal and human brain. Overall, ceperognastat had an acceptable safety profile in Phase 1 clinical studies with no serious adverse events reported following single and multiple dosing. The PK, enzyme occupancy, and safety profile supported Phase 2 development of ceperognastat.
Discussion: Ceperognastat is an orally available, highly potent, CNS-penetrant OGA inhibitor that achieved high (> 80%) OGA enzyme occupancy and increased brain O-GlcNAc-tau preclinically. Ceperognastat demonstrated > 95% OGA enzyme occupancy in Phase 1 trials. These occupancy data informed the dose selection for the Phase 2 clinical program.
Highlights: Ceperognastat is a highly potent, CNS-penetrant OGA inhibitor.Ceperognastat is both orally available and CNS-penetrant even when given at low doses.Ceperognastat can achieve > 95% OGA enzyme occupancy in the animal and human brain.Ceperognastat had an acceptable safety profile in Phase 1 clinical studies.
Keywords: Alzheimer's disease; O‐GlcNAcase inhibition; O‐GlcNAcylation; enzyme occupancy; pharmacokinetics; tau.
© 2024 Eli Lilly and Company and Invicro, LLC. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
William Kielbasa, Paul Goldsmith, Kevin B. Donnelly, Hugh N. Nuthall, Sergey Shcherbinin, Adam S. Fleisher, Jörg Hendle, Susan L. DuBois, Stephen L. Lowe, Feiyu Fred Zhang, Eric M. Woerly, Michele Mancini, Emily C. Collins, Miroslaw Brys, Michael L. Hutton and Dustin J. Mergott are employees and minor shareholders at Eli Lilly and Company. Nicolas J.‐F. Dreyfus, David Evans and Jeremy Gilmore were employed by Eli Lilly and Company during this work. Eli Lilly and Company are developing patents relevant to this research. David Evans is currently employed by Google DeepMind, has received Alphabet stock as part of this employment, and is an author on a published patent for Exscientia WO2022106857A1. Cristian C. Constantinescu and David S. Russell are current employees at Invicro, whereas Roger N. Gunn was an employee at Invicro during this work. Author disclosures are available in the Supporting Information.
Figures


References
-
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6(4):487‐498. - PubMed
-
- Selkoe DJ. In the beginning. Nature. 1991;354(6353):432‐433. - PubMed
-
- Bruée L, Bussière T, Bruée‐Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33(1):95‐130. - PubMed
LinkOut - more resources
Full Text Sources
