Mitochondria-Targeting Virus-Like Gold Nanoparticles Enhance Chemophototherapeutic Efficacy Against Pancreatic Cancer in a Xenograft Mouse Model
- PMID: 39748900
- PMCID: PMC11693971
- DOI: 10.2147/IJN.S497346
Mitochondria-Targeting Virus-Like Gold Nanoparticles Enhance Chemophototherapeutic Efficacy Against Pancreatic Cancer in a Xenograft Mouse Model
Abstract
Background: The dense and fibrotic nature of the pancreatic tumor microenvironment significantly contributes to tumor invasion and metastasis. This challenging environment acts as a formidable barrier, hindering effective drug penetration and delivery, which ultimately limits the efficacy of conventional cancer treatments. Gold nanoparticles (AuNPs) have emerged as promising nanocarriers to overcome the extracellular matrix barrier; however, their limited targeting precision, poor delivery efficiency, and insufficient photothermal conversion present challenges.
Methods: We developed triphenyl phosphonium-functionalized high-branch gold nanoparticles, denoted as Dox@TPAu, to enhance drug delivery and targeting capabilities. The targeted penetration, biopharmaceutical and pharmacokinetic properties of Dox@TPAu were characterized, and the synergistic therapeutic effect was evaluated by the BxPC-3 xenograft tumor mouse model.
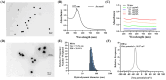
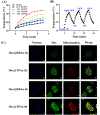
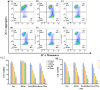
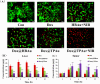
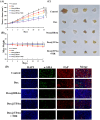
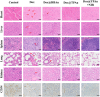
Results: Dox@TPAu exhibits superior photothermal conversion efficiency (91.0%) alongside a high drug loading efficiency (26%) and effective photo-triggered drug-release potential. This Dox@TPAu drug delivery system adeptly accumulates at tumor sites due to its unique properties, enabling targeted localization within cancer cells and the mitochondria of stromal fibroblasts. This localization disrupts mitochondrial function and transfer-processes crucial for energy production, metabolism, and cell signaling within the tumor microenvironment. Pharmacokinetic analyses revealed an optimal spatiotemporal distribution of Dox@TPAu at the tumor site. This strategic accumulation enables precise disruption of both the physical barrier and cancer cells, enhancing treatment efficacy through near-infrared light-triggered local chemo-photothermal synergistic therapy.
Conclusion: Our findings demonstrate that this innovative strategy effectively leverages the unique properties of mitochondria-targeting, virus-like AuNPs for precise and efficient stromal depletion, presenting a promising approach to enhance the efficacy of pancreatic cancer treatment.
Keywords: chemo-photothermal therapy; gold nanoparticle; mitochondria-targeting; pancreatic cancer; stromal depletion.
© 2024 Meng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

