Emerging Therapeutic Modalities and Pharmacotherapies in Neuropathic Pain Management: A Systematic Review and Meta-Analysis of Parallel Randomized Controlled Trials
- PMID: 39748928
- PMCID: PMC11695085
- DOI: 10.1155/prm/6782574
Emerging Therapeutic Modalities and Pharmacotherapies in Neuropathic Pain Management: A Systematic Review and Meta-Analysis of Parallel Randomized Controlled Trials
Abstract
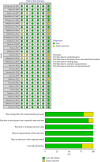
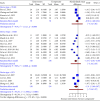
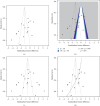
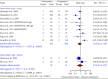
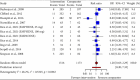
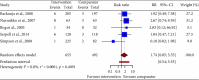
Background: Neuropathic pain (NP) is a chronic condition caused by abnormal neuronal excitability in the nervous system. Current treatments for NP are often ineffective or poorly tolerated. Hence, we reviewed the efficacy and safety of novel drugs or devices that target neuronal excitability in NP patients compared with placebo, sham, or usual care interventions. Methods: Six databases were searched for parallel randomized controlled trials (RCTs) reporting novel devices (rTMS, SCS, and TENS) or drugs (EMA401, capsaicin 8% patch, and Sativex) for NP. Data were extracted and quality was assessed using the ROB2 tool. The random-effects inverse variance method was used for analysis. Results: In our review of 30 RCTs with 4251 participants, device-based interventions were found to be more effective in reducing pain scores than control interventions (SMD = -1.27, 95% CI: -1.92 to -0.62). However, high heterogeneity was seen (p < 0.01, I 2 = 91%), attributable to the etiology of NP (R 2 = 58.84%) and year of publication (R 2 = 49.49%). Funding source and type of control comparator were ruled out as cause of heterogeneity. Although drug interventions did not differ from placebo interventions in absolute pain reduction (SMD = -1.21, 95% CI: -3.55 to 1.13), when comparing relative change in pain intensity from baseline, drug interventions were found to be effective (SMD = 0.29, 95% CI: 0.04-0.55). Asymmetry in the funnel plot was visualized, suggesting publication bias. Certainty of evidence was very low according to GRADE assessment. Conclusions: Our review indicates that device-based interventions are more effective than control interventions in reducing pain intensity in NP. Nevertheless, available evidence is limited due to heterogeneity and publication bias, prompting the need for more high-quality RCTs to confirm the efficacy and safety of these interventions.
Keywords: EMA401; Sativex; capsaicin; neuropathic pain; spinal cord stimulation; transcranial magnetic stimulation; transcutaneous electrical nerve stimulation.
Copyright © 2024 Ernest Kissi Kontor et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

