Using multiple drug similarity networks to promote adverse drug event detection
- PMID: 39748955
- PMCID: PMC11693886
- DOI: 10.1016/j.heliyon.2024.e39728
Using multiple drug similarity networks to promote adverse drug event detection
Abstract
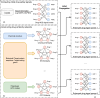
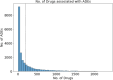
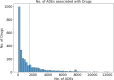
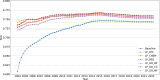
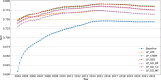
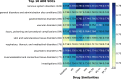
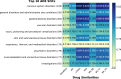
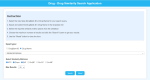
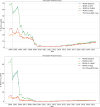
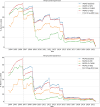
The occurrence of an adverse drug event (ADE) has become a serious social concern of public health. Early detection of ADEs can lower the risk of drug safety as well as the expense of the drug. While post-market spontaneous reports of ADEs remain a cornerstone of pharmacovigilance, most existing signal detection algorithms rely on substantial accumulated data, limiting their applicability to early ADE detection when reports are scarce. To address this issue, we propose a label propagation model for generating enhanced drug safety signals using multiple drug features. We first construct multiple drug similarity networks using a range of drug features. We then calculate initial drug safety signals using conventional signal detection algorithms. These original signals are subsequently propagated across each drug similarity network to obtain enhanced drug safety signals. We evaluate our proposed model using two common signal detection algorithms on data from the FDA Adverse Event Reporting System (FAERS). Results demonstrate that enhanced drug safety signals with pre-clinical information outperform the standard safety signal detection algorithms on early ADE detection. In addition, we systematically evaluate the performance of different drug similarities against different types of ADEs. Furthermore, we have developed a web interface (http://drug-drug-sim.aimedlab.net/) to display our multiple drug similarity scores, facilitating access to this valuable resource for drug safety monitoring.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Lazarou J., Pomeranz B.H., Corey P.N. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Rosen A.C., et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am. J. Clin. Dermatol. 2013;14:327–333. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

