Cytotoxic and proliferation-inhibitory activity of natural and synthetic fungal tropolone sesquiterpenoids in various cell lines
- PMID: 39748960
- PMCID: PMC11693898
- DOI: 10.1016/j.heliyon.2024.e37713
Cytotoxic and proliferation-inhibitory activity of natural and synthetic fungal tropolone sesquiterpenoids in various cell lines
Abstract
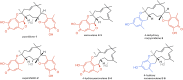
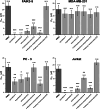
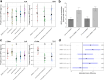
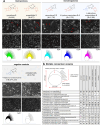
Fungal specialized metabolites are known for their potent biological activities, among which tropolone sesquiterpenoids (TS) stand out for their diverse bioactivities. Here, we report cytotoxic and proliferation inhibitory effects of the recently discovered TS compounds 4-hydroxyxenovulene B and 4-dihydroxy norpycnidione, and the structurally related 4-hydroxy norxenovulene B and xenovulene B. Inhibition of metabolic activity after TS treatment was observed in Jurkat, PC-3 and FAIK3-5 cells, whereas MDA-MB-231 cells were unresponsive to treatment. Structurally similar epolones were shown to induce erythropoietin (EPO). Therefore, FAIK3-5 cells, which can naturally produce EPO, were applied to test the compounds in this regard. While no effect on EPO production in FAIK3-5 cells could be demonstrated, effects on their proliferation, viability, and morphology were observed depending on the presence of tropolone moieties in the molecules. Our study underlines the importance of relevant cell models for bioactivity testing of compounds with unknown mechanisms of action.
Keywords: Bioactivity; Cytotoxicity; Erythropoietin; Proliferation; Synthetic biology; Tropolone sesquiterpenoids.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Synthetic Biology Driven Biosynthesis of Unnatural Tropolone Sesquiterpenoids.Angew Chem Int Ed Engl. 2020 Dec 21;59(52):23870-23878. doi: 10.1002/anie.202009914. Epub 2020 Oct 26. Angew Chem Int Ed Engl. 2020. PMID: 32929811 Free PMC article.
-
Tropolones and Thailandepsin B as Lead-like Natural Compounds in the Development of Potent and Selective Histone Deacetylase Inhibitors.Curr Drug Targets. 2023;24(9):698-717. doi: 10.2174/1389450124666230707144251. Curr Drug Targets. 2023. PMID: 37424350
-
Semisynthetic derivatives of the fungal metabolite eupenifeldin via targeting the tropolone hydroxy groups.Bioorg Med Chem Lett. 2024 Sep 15;110:129875. doi: 10.1016/j.bmcl.2024.129875. Epub 2024 Jul 2. Bioorg Med Chem Lett. 2024. PMID: 38964520
-
Stereochemical and Biosynthetic Rationalisation of the Tropolone Sesquiterpenoids.J Fungi (Basel). 2022 Aug 31;8(9):929. doi: 10.3390/jof8090929. J Fungi (Basel). 2022. PMID: 36135654 Free PMC article. Review.
-
Fungal Bergamotane Sesquiterpenoids-Potential Metabolites: Sources, Bioactivities, and Biosynthesis.Mar Drugs. 2022 Dec 8;20(12):771. doi: 10.3390/md20120771. Mar Drugs. 2022. PMID: 36547918 Free PMC article. Review.
References
-
- Peláez F. In: Handbook of Industrial Mycology. An Z., editor. M. Dekker; 2005. Biological activities of fungal metabolites; pp. 49–92.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

