Biomarkers for immunotherapy resistance in non-small cell lung cancer
- PMID: 39749035
- PMCID: PMC11693593
- DOI: 10.3389/fonc.2024.1489977
Biomarkers for immunotherapy resistance in non-small cell lung cancer
Abstract
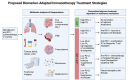
Immunotherapy has revolutionised the treatment landscape of non-small cell lung cancer (NSCLC), significantly improving survival outcomes and offering renewed hope to patients with advanced disease. However, the majority of patients experience limited long-term benefits from immune checkpoint inhibition (ICI) due to the development of primary or acquired immunotherapy resistance. Accurate predictive biomarkers for immunotherapy resistance are essential for individualising treatment strategies, improving survival outcomes, and minimising potential treatment-related harm. This review discusses the mechanisms underlying resistance to immunotherapy, addressing both cancer cell-intrinsic and cancer cell-extrinsic resistance processes. We summarise the current utility and limitations of two clinically established biomarkers: programmed death ligand 1 (PD-L1) expression and tumour mutational burden (TMB). Following this, we present a comprehensive review of emerging immunotherapy biomarkers in NSCLC, including tumour neoantigens, epigenetic signatures, markers of the tumour microenvironment (TME), genomic alterations, host-microbiome composition, and circulating biomarkers. The potential clinical applications of these biomarkers, along with novel approaches to their biomarker identification and targeting, are discussed. Additionally, we explore current strategies to overcome immunotherapy resistance and propose incorporating predictive biomarkers into an adaptive clinical trial design, where specific immune signatures guide subsequent treatment selection.
Keywords: biomarkers; circulating biomarkers; immunotherapy resistance; non-small cell lung cancer (NSCLC); precision oncology strategies.
Copyright © 2024 Rother, John and Wong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer.Cancer Med. 2021 Apr;10(7):2216-2231. doi: 10.1002/cam4.3649. Epub 2021 Mar 2. Cancer Med. 2021. PMID: 33655698 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
-
Harnessing the epigenome to boost immunotherapy response in non-small cell lung cancer patients.Ther Adv Med Oncol. 2021 May 25;13:17588359211006947. doi: 10.1177/17588359211006947. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34104224 Free PMC article. Review.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
Cited by
-
Lung Stereotactic Body Radiotherapy (SBRT): Challenging Scenarios and New Frontiers.J Clin Med. 2025 Jul 9;14(14):4871. doi: 10.3390/jcm14144871. J Clin Med. 2025. PMID: 40725565 Free PMC article. Review.
-
Resistance in Lung Cancer Immunotherapy and How to Overcome It: Insights from the Genetics Perspective and Combination Therapies Approach.Cells. 2025 Apr 12;14(8):587. doi: 10.3390/cells14080587. Cells. 2025. PMID: 40277912 Free PMC article. Review.
-
Prognostic value of the systemic immune-inflammation index in non-small cell lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis.Front Oncol. 2025 May 16;15:1532343. doi: 10.3389/fonc.2025.1532343. eCollection 2025. Front Oncol. 2025. PMID: 40452837 Free PMC article.
-
Immunotherapy resistance in non-small cell lung cancer: from mechanisms to therapeutic opportunities.J Exp Clin Cancer Res. 2025 Aug 23;44(1):250. doi: 10.1186/s13046-025-03519-z. J Exp Clin Cancer Res. 2025. PMID: 40849659 Free PMC article. Review.
-
Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study.Cancers (Basel). 2025 Jul 18;17(14):2384. doi: 10.3390/cancers17142384. Cancers (Basel). 2025. PMID: 40723266 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

