Real-World-Data of Treatment-Naïve and Previously Treated Patients Receiving Up to 3 Injections of Faricimab in Neovascular Age-Related Macular Degeneration
- PMID: 39749039
- PMCID: PMC11694016
- DOI: 10.2147/OPTH.S482948
Real-World-Data of Treatment-Naïve and Previously Treated Patients Receiving Up to 3 Injections of Faricimab in Neovascular Age-Related Macular Degeneration
Abstract
Purpose: To evaluate visual and anatomical outcome of consecutive patients who received intravitreal injections (IVI) of faricimab for the treatment of neovascular age-related macular degeneration (nAMD).
Patients and methods: A retrospective study of patients treated for nAMD with one to three IVIs of faricimab from October 2022 to January 2024. Demographic data, treatment history, best corrected visual acuity (BCVA), anatomic parameters, and adverse events (AEs) were collected.
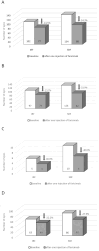
Results: After one IVI of faricimab, previously treated (n=160) eyes with a mean of 33.51 IVIs and treatment-naïve (n=10) eyes showed a mean BCVA gain of +0.59±0.52 letters (p=0.364) and +5.00±6.50 letters (p=0.461), respectively, and a mean central subfield thickness (CST) reduction of -27.65±5.33 µm (p<0.001) and -94.10±39.74 μm (p=0.042), respectively. In treatment-refractory eyes after switching from aflibercept (n=108), mean BCVA increased by +0.42±0.66 (p=0.745) and the mean CST improved by -21.98±6.04 (p<0.001). After three IVIs of faricimab previously treated (n=106) and treatment-naïve (n=5) eyes showed a mean BCVA increase of +1.57±0.88 letters (p=0.051) versus +12.50±8.14 letters (p=0.185), and a mean CST reduction of -25.51±5.82 µm (p<0.001) versus -82.60±36.20 µm from baseline, respectively. In treatment-refractory eyes after switching from aflibercept (n=79), mean BCVA improved by +2.15±1.08 letters (p=0.029) and mean CST decreased by -27.46±7.04 µm (p<0.001). Mean pigment epithelial detachment (PED) was also significantly reduced even between the first and the third faricimab injection in previously treated eyes (p=0.03). The proportion of eyes with intraretinal fluid and subretinal fluid improved significantly in all eyes and treatment-refractory eyes after switching from aflibercept. Ocular AEs were reported in three out of 170 eyes, and one patient had two stroke events during faricimab therapy.
Conclusion: Three IVIs of faricimab have the potential to improve visual acuity and anatomical parameters even in treatment-refractory nAMD eyes with a mean dosing interval of more than 6 weeks.
Keywords: Vabysmo; anti-vascular endothelial growth factor; intravitreal dosing interval; intravitreal injection; treatment resistant nAMD.
© 2024 Kunzmann et al.
Conflict of interest statement
Dr Berenike Kunzmann is speaker for Roche. Prof Dr med Karl Ulrich Bartz-Schmidt has received consulting and speaker fees from Roche, Pixium, Alexion Pharmaceuticals Inc., Breye Therapeutics, Gyroscope Therapeutics, Apellis Pharmaceuticals Inc., Janssen-Cilag GmbH, Pixium Vision, ViGeneron GmbH, Biogen Inc., CureVac med. PD Dr med Bianka Sobolewska has received a travel grant from Galderma, Novartis, and Santen. Dr Alexandra Schweig reports Advisory Board non-personal fees from Bayer and Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi:10.1016/S0140-6736(22)00010-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources

