Limitations of a proper SFTSV mouse model using human C-type lectin receptors
- PMID: 39749135
- PMCID: PMC11693710
- DOI: 10.3389/fmicb.2024.1452739
Limitations of a proper SFTSV mouse model using human C-type lectin receptors
Abstract
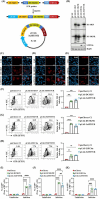
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tick-borne virus with a human mortality rate of up to 30%, posing a significant threat to public health. However, the lack of suitable research models has impeded the development of effective human vaccines. In this study, we engineered transgenic mice (3xTg) using a novel construct that simultaneously expresses three C-type Lectin receptors, identified as critical SFTSV entry receptors. While this construct substantially enhanced viral binding and infection in BJAB cells, the 3xTg mice exhibited only limited SFTSV replication in the lymph nodes and spleen, without significant impacts on morbidity or mortality. These findings highlight that the overexpression of entry receptors alone is insufficient to fully recapitulate human SFTSV infection in mice. Moreover, our results reveal that the introduction of multiple entry receptors does not necessarily translate to enhanced infection efficacy. This underscores the need for further investigation into the interplay between SFTSV entry mechanisms and host factors to develop more robust mouse models. Advancing such models will be crucial for unraveling the pathogenesis of SFTS pathology and improving strategies for its prevention and treatment in humans.
Keywords: DC-SIGN; DC-SIGNR; LSECtin; severe fever thrombocytopenia syndrome virus; transgenic mice.
Copyright © 2024 Kim, Ro, Lee, Song, Lee and Cho.
Conflict of interest statement
JL and HL Lee were employed by GEMCRO, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Characterization of Glycoprotein-Mediated Entry of Severe Fever with Thrombocytopenia Syndrome Virus.J Virol. 2016 May 12;90(11):5292-5301. doi: 10.1128/JVI.00110-16. Print 2016 Jun 1. J Virol. 2016. PMID: 26984731 Free PMC article.
-
Modeling Severe Fever with Thrombocytopenia Syndrome Virus Infection in Golden Syrian Hamsters: Importance of STAT2 in Preventing Disease and Effective Treatment with Favipiravir.J Virol. 2017 Jan 18;91(3):e01942-16. doi: 10.1128/JVI.01942-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881648 Free PMC article.
-
Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines.J Virol. 2013 Apr;87(8):4384-94. doi: 10.1128/JVI.02628-12. Epub 2013 Feb 6. J Virol. 2013. PMID: 23388721 Free PMC article.
-
A new emerging pandemic of severe fever with thrombocytopenia syndrome (SFTS).Virusdisease. 2021 Jun;32(2):220-227. doi: 10.1007/s13337-021-00656-9. Epub 2021 Apr 29. Virusdisease. 2021. PMID: 33942022 Free PMC article. Review.
-
Unraveling the Underlying Interaction Mechanism Between Dabie bandavirus and Innate Immune Response.Front Immunol. 2021 May 27;12:676861. doi: 10.3389/fimmu.2021.676861. eCollection 2021. Front Immunol. 2021. PMID: 34122440 Free PMC article. Review.
References
-
- Brown G. D., Willment J. A., Whitehead L. (2018). C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 18 374–389. - PubMed
LinkOut - more resources
Full Text Sources

