Dosing Regimen Recommendations for Sirolimus in Adult Liver Transplant Recipients: Insights from a Population Pharmacokinetic Model
- PMID: 39749189
- PMCID: PMC11693943
- DOI: 10.2147/DDDT.S503463
Dosing Regimen Recommendations for Sirolimus in Adult Liver Transplant Recipients: Insights from a Population Pharmacokinetic Model
Abstract
Background: Sirolimus is a commonly used immunosuppressant administered after solid organ transplantation. It is characterized by a narrow therapeutic window and highly variable exposure, necessitating the identification of the sources of variability and design of individualized drug therapies.
Aim: This study aimed to perform a population pharmacokinetic (PK) analysis of sirolimus in adult liver transplant recipients and develop dosing regimen recommendations according to patient characteristics.
Methodology: A total of 216 measurements of whole blood sirolimus concentrations in 103 adult patients were obtained for analysis. Covariates influencing the PKs of sirolimus were investigated using a stepwise procedure. Monte Carlo simulations were conducted to recommend dosing regimens for patients with different levels of covariates.
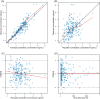
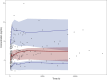
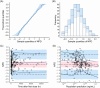
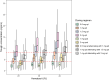
Results: A one-compartment model with first-order elimination provided the best fit of the data. Hematocrit (HCT) significantly influenced the apparent clearance of sirolimus. Monte Carlo simulations showed that for patients with a low HCT level of 28%, dosing regimens of 1.5 mg qd or 1 mg qd alternating with 1.5 mg qd should be recommended. For patients with a normal HCT level, the recommended dosing regimens were 1 mg qd, 2 mg qod, or 0.5 mg qd alternating with 1 mg qd.
Conclusion: Based on our population PK model of sirolimus in adult liver transplant recipients, which has the largest sample size to date, we recommend to tailor dosing regimens to various HCT levels in such patients.
Keywords: dosing regimen; hematocrit; liver transplant; population pharmacokinetic analysis; sirolimus.
© 2024 Mao et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Population pharmacokinetic analysis and dosing guidelines for tacrolimus co-administration with Wuzhi capsule in Chinese renal transplant recipients.J Clin Pharm Ther. 2021 Aug;46(4):1117-1128. doi: 10.1111/jcpt.13407. Epub 2021 Mar 25. J Clin Pharm Ther. 2021. PMID: 33768546
-
Limited sampling models and Bayesian estimation for mycophenolic acid area under the curve prediction in stable renal transplant patients co-medicated with ciclosporin or sirolimus.Clin Pharmacokinet. 2009;48(11):745-58. doi: 10.2165/11318060-000000000-00000. Clin Pharmacokinet. 2009. PMID: 19817503
-
Model-Informed individualized dosage regimen of sirolimus in pediatric patients with intractable lymphatic malformations.Eur J Pharm Sci. 2024 Sep 1;200:106837. doi: 10.1016/j.ejps.2024.106837. Epub 2024 Jul 1. Eur J Pharm Sci. 2024. PMID: 38960206
-
Safety and efficacy of TOR inhibitors in pediatric renal transplant recipients.Am J Kidney Dis. 2001 Oct;38(4 Suppl 2):S22-8. doi: 10.1053/ajkd.2001.27838. Am J Kidney Dis. 2001. PMID: 11583941 Review.
-
Immunosuppressive drug monitoring of sirolimus and cyclosporine in pediatric patients.Clin Biochem. 2004 Jun;37(6):424-8. doi: 10.1016/j.clinbiochem.2004.04.001. Clin Biochem. 2004. PMID: 15183289 Review.
References
-
- Sehgal S. Sirolimus: a new immunosuppressive agent: a historical perspective and immunosuppressive profile. In: Principles of Drug Development in Transplantation and Autoimmunity. Austin, TX: RG Landes; 1996:271–282.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

