Qingre Huoxue Decoction Alleviates Atherosclerosis by Regulating Macrophage Polarization Through Exosomal miR-26a-5p
- PMID: 39749190
- PMCID: PMC11693966
- DOI: 10.2147/DDDT.S487476
Qingre Huoxue Decoction Alleviates Atherosclerosis by Regulating Macrophage Polarization Through Exosomal miR-26a-5p
Abstract
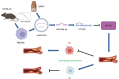
Background: Qingre Huoxue Decoction (QRHX) is a classical Chinese herbal prescription widely used in clinical practice for the treatment of atherosclerosis (AS). Our previous study demonstrated its efficacy in stabilizing plaque and improving prognosis, as well as its ability to regulate macrophage polarization. This study aimed to further investigate the effects of QRHX on AS and explore the underlying mechanisms.
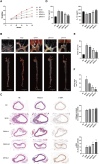
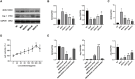
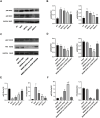
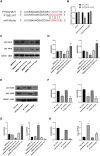
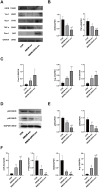
Methods: ApoE-/- mice were fed a high-fat diet (HFD) for 8 weeks in order to establish an AS model. Oil Red O, H&E, Masson, and IHC staining were employed to assess lipid accumulation, plaque development, collagen loss and target of the aortas tissue. ELISA was employed to measure the levels of TNF-α and IL-10 in serum. Dual luciferase reporter assay was conducted to ascertain the connection between miR-26a-5p and PTGS2 in vitro. Western blot and RT-qPCR assay were conducted to assess the NF-κB signaling pathway and macrophage polarization. The effects of miR-26a-5p were tested after transfecting miR-26a-5p over-expressive lentivirus.
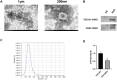
Results: QRHX attenuated HFD-induced plaque progression and inflammation of AS model mice. BMDM-derived exosomes (BMDM-exo) increased miR-26a-5p and decreased PTGS2 expressions, inhibited the NF-κB signaling pathway and regulated macrophage polarization in vivo. These effects of BMDM-exo were further enhanced after QRHX intervention. Dual luciferase reporter assay results showed that miR-26a-5p directly binds to the 3'-UTR of PTGS2 mRNA and regulates the expression of PTGS2. The miR-26a-5p of BMDM-exo played a key role in macrophage polarization. After overexpression of miR-26a-5p, the NF-κB signaling pathway was inhibited and macrophages were converted from M1 to M2 in vitro.
Conclusion: QRHX can exert anti-inflammatory and plaque-stabilizing effects through exosomal miR-26a-5p via inhibiting the PTGS2/NF-κB signaling pathway and regulating macrophage phenotype from M1 to M2 polarization in AS.
Keywords: Qingre Huoxue decoction; atherosclerosis; exosomes; macrophage polarization; miR-26a-5p.
© 2024 He et al.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures

Similar articles
-
Qingre Huoxue Decoction regulates macrophage polarisation to attenuate atherosclerosis through the inhibition of NF-κB signalling-mediated inflammation.J Ethnopharmacol. 2023 Jan 30;301:115787. doi: 10.1016/j.jep.2022.115787. Epub 2022 Oct 4. J Ethnopharmacol. 2023. PMID: 36206868
-
Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis.Acta Biochim Biophys Sin (Shanghai). 2021 Aug 31;53(9):1227-1236. doi: 10.1093/abbs/gmab102. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34350954
-
Exosome microRNA-125a-5p derived from epithelium promotes M1 macrophage polarization by targeting IL1RN in chronic obstructive pulmonary disease.Int Immunopharmacol. 2024 Aug 20;137:112466. doi: 10.1016/j.intimp.2024.112466. Epub 2024 Jun 13. Int Immunopharmacol. 2024. PMID: 38875998
-
[Buyang Huanwu Decoction delays vascular aging in rats through exosomal miR-590-5p signal-mediated macrophage polarization].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Jun 20;45(6):1251-1259. doi: 10.12122/j.issn.1673-4254.2025.06.14. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40579138 Free PMC article. Chinese.
-
Platelet-derived exosomes regulate endothelial cell inflammation and M1 macrophage polarization in coronary artery thrombosis via modulating miR-34a-5p expression.Sci Rep. 2024 Jul 29;14(1):17429. doi: 10.1038/s41598-024-67654-x. Sci Rep. 2024. PMID: 39075107 Free PMC article.
Cited by
-
The NLRP3 inflammasome: a therapeutic target of phytochemicals in treating atherosclerosis (a systematic review).Front Immunol. 2025 May 15;16:1568722. doi: 10.3389/fimmu.2025.1568722. eCollection 2025. Front Immunol. 2025. PMID: 40443656 Free PMC article.
-
FOS as a biomarker for myocardial infarction treatment with Deng's Yangxin Decoction: a systems biology-based analysis.Front Cardiovasc Med. 2025 May 30;12:1488684. doi: 10.3389/fcvm.2025.1488684. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40520937 Free PMC article.
-
Risk Warning of Neutrophil-to-Lymphocyte Ratio for Neurological Recovery in Acute Ischemic Stroke After Thrombolysis: A Retrospective Study.Brain Behav. 2025 Mar;15(3):e70373. doi: 10.1002/brb3.70373. Brain Behav. 2025. PMID: 40022214 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

