Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile
- PMID: 39749348
- PMCID: PMC11693714
- DOI: 10.3389/fimmu.2024.1414304
Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile
Abstract
Background: Biosimilar natalizumab (biosim-NTZ) is the first biosimilar monoclonal antibody of reference natalizumab (ref-NTZ) for treatment of relapsing forms of multiple sclerosis (MS). Within the totality of evidence for demonstration of biosimilarity, immunogenicity assessments were performed in healthy subjects and patients with relapsing-remitting MS (RRMS) to confirm a matching immunogenicity profile between biosim-NTZ and ref-NTZ.
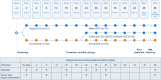
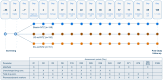
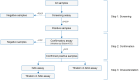
Methods: Immunogenicity of biosim-NTZ versus ref-NTZ was evaluated in two pivotal clinical studies. In a comparative efficacy and safety study, patients with RRMS (n=264) received monthly infusions of biosim-NTZ/EU-ref-NTZ over 48 weeks. The primary endpoint period was Week 0 to Week 24. In a separate, comparative pharmacokinetic/pharmacodynamic (PK/PD) study, healthy subjects (n=450) received a single dose of biosim-NTZ, US-ref-NTZ or EU-ref-NTZ prior to an 85-day follow-up. In both studies, state-of-the-art, highly sensitive and drug tolerant bioanalytical assays were used to identify the proportion of participants with anti-drug antibodies (ADA) and neutralizing antibodies (NAb) against natalizumab over time.
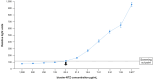
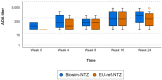
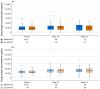
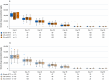
Results: In the comparative efficacy and safety study, biosim-NTZ and EU-ref-NTZ demonstrated similar incidences of overall ADA (79.4% vs 73.7%, respectively) and NAb (68.7% vs 66.2%, respectively) at Week 24. ADA titers over time were also concordant throughout the study period. Switching treatment from EU-ref-NTZ to biosim-NTZ had no impact on treatment-related ADA/NAb or clinical responses. Likewise, the single-dose PK/PD study reported matching overall incidence of ADA between treatment groups and matching ADA titer profiles over time.
Conclusion: The immunogenicity profile of biosim-NTZ was confirmed to match that of ref-NTZ in healthy subjects and patients with RRMS by applying highly sensitive methods.
Keywords: anti-drug antibodies; biologic products; biosimilar; immunogenicity; immunology; multiple sclerosis; natalizumab; neutralizing antibodies.
Copyright © 2024 Chamberlain, Hemmer, Höfler, Wessels, von Richter, Hornuss, Poetzl and Roth.
Conflict of interest statement
PC was employed by bioLOGICA Consulting. JH was employed by Staburo GmbH. HW and KR were employed Polpharma Biologics S.A. OvR, CH and JP were employed by Hexal AG (a Sandoz company). PC has received consulting fees from Polpharma Biologics S.A. for scientific and regulatory consulting related to PB006. BH has served on scientific advisory boards for Novartis; he has served as a DMSC member for AllergyCare, Polpharma, Sandoz, and TG therapeutics; and he has received honoraria for counseling from the Gerson Lehrman Group. He or his institution has received speaker honoraria from Desitin; his institution has received research grants from Regeneron and Hoffmann-La-Roche for multiple sclerosis research. BH holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralizing antibodies to interferon. All of BH’s conflicts are not relevant to the topic of the study. BH has received funding from the Multiple MS EU consortium and WISDOM EU consortia, the Clinspect-M consortium funded by the Bundesministerium für Bildung und Forschung, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology EXC 2145 SyNergy – ID 390857198. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Polpharma Biologics S.A. The funder had the following involvement in the study: PB006-03-01 Antelope study (NCT04115488) and the PB006-01-03 PK/PD study (EudraCT system: 2019-003874-15).
Figures
References
-
- Chamberlain P, Kurki P. Immunogenicity assessment of biosimilars: a multidisciplinary perspective. Cham, Switzerland: Springer International Publishing; (2018) p. 489–542. doi: 10.1007/978-3-319-99680-6_19 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

