The interaction between nirmatrelvir/ritonavir and sirolimus: a case report of a kidney recipient with renal insufficiency and COVID-19
- PMID: 39749601
- PMCID: PMC11700402
- DOI: 10.1177/03000605241307845
The interaction between nirmatrelvir/ritonavir and sirolimus: a case report of a kidney recipient with renal insufficiency and COVID-19
Abstract
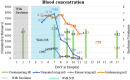
Nirmatrelvir/ritonavir is a novel drug combination authorized by the US Food and Drug Administration for the treatment of coronavirus disease 2019 (COVID-19). This report describes the case of a patient with a prior history of kidney transplantation who received nirmatrelvir/ritonavir. In this case, sirolimus use was successfully stopped before nirmatrelvir/ritonavir treatment, and the nirmatrelvir/ritonavir trough concentration was determined. During nirmatrelvir/ritonavir treatment, the sirolimus trough concentration remained stable. This case highlights the risk associated with the concomitant administration of sirolimus and nirmatrelvir/ritonavir. Providers should therefore be cautious when prescribing nirmatrelvir/ritonavir to kidney transplant recipients currently receiving sirolimus, with caution exercised based on creatinine clearance.
Keywords: COVID-19; Nirmatrelvir/ritonavir; drug–drug interaction; immunosuppression; kidney transplantation; sirolimus.
Figures
Similar articles
-
Nirmatrelvir/ritonavir treatment of patients with COVID-19 taking tacrolimus: case series describing the results of drug-drug interactions.J Int Med Res. 2024 May;52(5):3000605241247705. doi: 10.1177/03000605241247705. J Int Med Res. 2024. PMID: 38698526 Free PMC article.
-
Management and Outcome of COVID-19 Infection Using Nirmatrelvir/Ritonavir in Kidney Transplant Patients.Clin J Am Soc Nephrol. 2023 Jul 1;18(7):913-919. doi: 10.2215/CJN.0000000000000186. Epub 2023 Apr 26. Clin J Am Soc Nephrol. 2023. PMID: 37099447 Free PMC article.
-
"Saving lives with nirmatrelvir/ritonavir one transplant patient at a time".Transpl Infect Dis. 2023 Apr;25(2):e14037. doi: 10.1111/tid.14037. Epub 2023 Feb 27. Transpl Infect Dis. 2023. PMID: 36847419 Free PMC article. Review.
-
Nirmatrelvir/ritonavir treatment in SARS-CoV-2 positive kidney transplant recipients - a case series with four patients.BMC Nephrol. 2023 Apr 15;24(1):99. doi: 10.1186/s12882-023-03154-w. BMC Nephrol. 2023. PMID: 37061677 Free PMC article.
-
Optimizing the use of nirmatrelvir/ritonavir in solid organ transplant recipients with COVID-19: A review of immunosuppressant adjustment strategies.Front Immunol. 2023 Apr 4;14:1150341. doi: 10.3389/fimmu.2023.1150341. eCollection 2023. Front Immunol. 2023. PMID: 37081880 Free PMC article. Review.
References
-
- Gagnier JJ, Kienle G, Altman DG; CARE Group et al.. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. - PubMed
-
- Dong R, Huang Y, Ling X, et al.. High concentrations of nirmatrelvir/ritonavir in critically ill patients receiving continuous renal replacement therapy. Int J Antimicrob Agents 2024; 63: 106997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical