SUCLG1 promotes aerobic respiration and progression in plexiform neurofibroma
- PMID: 39749698
- PMCID: PMC11753773
- DOI: 10.3892/ijo.2024.5716
SUCLG1 promotes aerobic respiration and progression in plexiform neurofibroma
Abstract
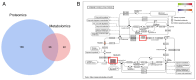
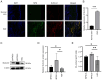
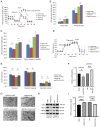
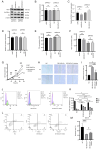
Plexiform neurofibromas (PNFs) are benign tumors that affect 20‑50% of patients with type I neurofibromatosis (NF1). PNF carries a risk of malignancy. There is no effective cure for PNF. Its onset may be associated with genetic and metabolic abnormalities, but the exact mechanisms remain unclear. Succinate‑CoA ligase GDP/ADP‑Forming Subunit α(SUCLG1), a catalytic enzyme in the tricarboxylic acid cycle, is highly expressed in PNF. The present study aimed to explore the role of SUCLG1 in function and metabolism of PNF cells. SUCLG1 expression was verified using western blotting and immunofluorescence. After inducing SUCLG1 knockdown and overexpression, functional changes in PNF cells were assessed, as well as effects of SUCLG1 on cell respiration and glucose metabolism. Quantitative PCR, WB, electron microscopy and Flow cytometry demonstrated that SUCLG1 enhanced mitochondrial quality and promoted mitochondrial fusion, thereby driving proliferation and migration of tumor cells, inhibiting apoptosis and altering the cell cycle. A Seahorse assay showed that elevated SUCLG1 expression enhanced cell aerobic respiration without affecting the glycolytic process. This suggests that SUCLG1 upregulation in PNF does not trigger the Warburg effect associated with malignant tumors. This study also demonstrated the positive regulation of cellular function by promoting the expression level of the SLC25A1 gene when SUCLG1 expression was elevated. In conclusion, SUCLG1 altered the mechanism of mitochondrial quality control to enhance cell aerobic respiration, thereby driving the pathogenesis of PNF. Thus, SUCLG1 can serve as a potential target in future therapeutic strategies.
Keywords: aerobic respiration; mitochondria; neurofibromatosis type 1; plexiform neurofibroma; tricarboxylic acid cycle.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Gross AM, Glassberg B, Wolters PL, Dombi E, Baldwin A, Fisher MJ, Kim A, Bornhorst M, Weiss BD, Blakeley JO, et al. Selumetinib in children with neurofibromatosis type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity. Neuro Oncol. 2022;24:1978–1988. doi: 10.1093/neuonc/noac109. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

