Photoresponsive Helical Foldamers: Conformational Control Through Double Helix Formation and Light-Induced Protonation
- PMID: 39749723
- PMCID: PMC11840663
- DOI: 10.1002/chem.202403771
Photoresponsive Helical Foldamers: Conformational Control Through Double Helix Formation and Light-Induced Protonation
Abstract
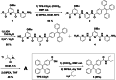
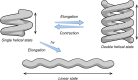
Helical foldamers constitute particularly relevant targets in the field of host-guest chemistry, be that as hosts or substrates. In this context, the strategies reported so far to control the dimensions and shape of foldamers mainly involve modifications of the skeleton through covalent synthesis. Herein, we prepared an oligopyridine dicarboxamide foldamer substituted by photo-active tetraphenylethylene units (TPE). We demonstrate that it is possible to toggle the length of a helical foldamer by two means. First, the elongation of foldamers can be tuned by adjusting the concentration, as demonstrated by DOSY NMR spectroscopy and X-ray diffraction analyses on both the single and the double helix structures. Secondly, and in a more original manner, a photo-induced protonation process triggered by TPE units promotes a novel pathway to unfold helical foldamers, leading to dramatic conformational and spectroscopic changes.
Keywords: Helical foldamers; conformational control; double helix formation; photoinduced process; tetraphenylethylene.
© 2025 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




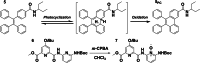


Similar articles
-
Light- and Temperature-Controlled Hybridization, Chiral Induction and Handedness of Helical Foldamers.Angew Chem Int Ed Engl. 2025 Jan 2;64(1):e202413629. doi: 10.1002/anie.202413629. Epub 2024 Oct 31. Angew Chem Int Ed Engl. 2025. PMID: 39225451 Free PMC article.
-
Macrocyclic Aromatic Amide Foldamer: Synthesis, Twisted-to-Boxlike Conformational Switching, and Molecular Recognition.Chempluschem. 2021 Jun 8;86(6):920-923. doi: 10.1002/cplu.202100193. Online ahead of print. Chempluschem. 2021. PMID: 34156762
-
Aromatic amide and hydrazide foldamer-based responsive host-guest systems.Acc Chem Res. 2014 Jul 15;47(7):1961-70. doi: 10.1021/ar5000242. Epub 2014 Mar 27. Acc Chem Res. 2014. PMID: 24673152
-
Stimuli-responsive synthetic helical polymers.Chem Soc Rev. 2024 Jan 22;53(2):793-852. doi: 10.1039/d3cs00952a. Chem Soc Rev. 2024. PMID: 38105704 Review.
-
Helical Anion Foldamers in Solution.Chem Rev. 2020 Mar 11;120(5):2759-2782. doi: 10.1021/acs.chemrev.9b00583. Epub 2020 Feb 10. Chem Rev. 2020. PMID: 32039583 Free PMC article. Review.
References
-
- Gellman S. H., Acc. Chem. Res. 1998, 31, 173–180.
-
- S. Hecht, I. Huc, Eds., Foldamers: Structure, Properties, and Applications, Wiley-VCH, Weinheim, 2007.
-
- Guichard G., Huc I., Chem. Commun. 2011, 47, 5933–5941. - PubMed
-
- Hill D. J., Mio M. J., Prince R. B., Hughes T. S., Moore J. S., Chem. Rev. 2001, 101, 3893–4012. - PubMed
-
- Yashima E., Ousaka N., Taura D., Shimomura K., Ikai T., Maeda K., Chem. Rev. 2016, 116, 13752–13990. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

