Role of the HGF/c-MET pathway in resistance to immune checkpoint inhibitors in advanced non-small cell lung cancer
- PMID: 39751636
- PMCID: PMC11698708
- DOI: 10.1007/s00262-024-03882-4
Role of the HGF/c-MET pathway in resistance to immune checkpoint inhibitors in advanced non-small cell lung cancer
Abstract
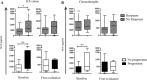
Most of advanced non-small cell lung cancer (NSCLC) patients will experience tumor progression with immunotherapy (IO). Preliminary data suggested an association between high plasma HGF levels and poor response to IO in advanced NSCLC. Our study aimed to evaluate further the role of the HGF/MET pathway in resistance to IO in advanced NSCLC. We included retrospectively 82 consecutive NSCLC patients from two academic hospitals. Among them, 49 patients received ICIs alone or in combination with chemotherapy (CT), while 33 patients received chemotherapy alone as the control group. We analyzed plasma HGF levels by ELISA and expression of PD-L1, MET/phospho-MET, and CD8+ T-Cell infiltration on lung tumor tissue by immunohistochemistry. We investigated the contribution of HGF/MET to IO response by culturing peripheral blood mononuclear cells (PBMC) with or without pembrolizumab, with recombinant HGF, or cocultured with NSCLC patients-derived explants. Additionally, c-MET inhibitors were used to evaluate the contribution of MET activation in NSCLC-mediated immunosuppression. High HGF levels were associated with high progression rate with IO (p = 0.0092), but not with CT. ELISA analysis of supernatants collected from cultured NSCLC cells showed that HGF was produced by tumor cells. Furthermore, when activated PBMCs were cultured in the presence of recombinant HGF or on NSCLC monolayer, the proliferation of CD3+CD8+ lymphocytes was inhibited, even in the presence of pembrolizumab. The addition of HGF/MET inhibitors restored lymphocyte activation and induced IFNγ production. In conclusion, inhibiting the HGF/MET signaling pathway could be a promising approach to enhance the efficacy of immunotherapy.
Keywords: HGF; MET; immunotherapy; non-small cell lung cancer; resistance.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Etienne Giroux Leprieur declares to have received honoraria/personal fees (advisory boards) from AstraZeneca, Bristol-Myers-Squibb MSD, and Roche. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ethical approval: Primary samples were collected at Ambroise Paré Hospital after obtaining signed informed consent (CPP IDF n°8) and stored within the Centre de Ressources Biologiques (CRB) of Ambroise Paré Hospital (ID CRB 2014-A00187-40).
Figures



References
-
- Pérez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, García-Aranda M, De Las Rivas J (2020) Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug Resist. Update Rev. Comment. Antimicrob. Anticancer Chemother. 53:100718. 10.1016/j.drup.2020.100718 - PubMed
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. 10.1056/NEJMoa1801005 - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. 10.1056/NEJMoa1606774 - PubMed
-
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. 10.1056/NEJMoa1810865 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

