Detecting somatic variants in purified brain DNA obtained from surgically implanted depth electrodes in epilepsy
- PMID: 39751777
- PMCID: PMC11997914
- DOI: 10.1111/epi.18251
Detecting somatic variants in purified brain DNA obtained from surgically implanted depth electrodes in epilepsy
Abstract
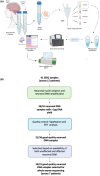
Objective: Somatic variants causing epilepsy are challenging to detect, as they are only present in a subset of brain cells (e.g., mosaic), resulting in low variant allele frequencies. Traditional methods relying on surgically resected brain tissue are limited to patients undergoing brain surgery. We developed an improved protocol to detect somatic variants using DNA from stereoelectroencephalographic (SEEG) depth electrodes, enabling access to a larger patient cohort and diverse brain regions. This protocol mitigates issues of contamination and low yields by purifying neuronal nuclei using fluorescence-activated nuclei sorting (FANS).
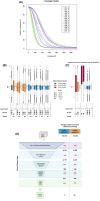
Methods: SEEG depth electrodes were collected upon extraction from 41 brain regions across 17 patients undergoing SEEG. Nuclei were isolated separately from depth electrodes in the affected brain regions (seizure onset zone) and the unaffected brain regions. Neuronal nuclei were isolated using FANS, and DNA was amplified using primary template amplification. Short tandem repeat (STR) analysis and postsequencing allelic imbalance assessment were used to evaluate sample integrity. High-quality amplified DNA samples from affected brain regions, patient-matched unaffected brain regions, and genomic DNA were subjected to whole exome sequencing (WES). A bioinformatic workflow was developed to reduce false positives and to accurately detect somatic variants in the affected brain region.
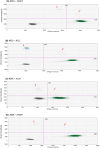
Results: Based on DNA yield and STR analysis, 14 SEEG-derived neuronal DNA samples (seven affected and seven unaffected) across seven patients underwent WES. From the variants prioritized using our bioinformatic workflow, we chose four candidate variants in MTOR, CSDE1, KLLN, and NLE1 across four patients based on pathogenicity scores and association with phenotype. All four variants were validated using digital droplet polymerase chain reaction.
Significance: Our approach enhances the reliability and applicability of SEEG-derived DNA for epilepsy, offering insights into its molecular basis, facilitating epileptogenic zone identification, and advancing precision medicine.
Keywords: DNA; depth electrodes; epilepsy; somatic variant; stereo‐EEG.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 2015;21:395–400. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

