First-line immune-based combinations or sunitinib in favorable-risk metastatic renal cell carcinoma: a real-world retrospective comparison from the ARON-1 study
- PMID: 39752009
- PMCID: PMC11699065
- DOI: 10.1007/s00262-024-03897-x
First-line immune-based combinations or sunitinib in favorable-risk metastatic renal cell carcinoma: a real-world retrospective comparison from the ARON-1 study
Abstract
Introduction: Renal cell carcinoma (RCC) is one of the most common types of urogenital cancer. The introduction of immune-based combinations, including dual immune-checkpoint inhibitors (ICI) or ICI plus tyrosine kinase inhibitors (TKIs), has radically changed the treatment landscape for metastatic RCC, showing varying efficacy across different prognostic groups based on the International Metastatic RCC Database Consortium (IMDC) criteria.
Materials and methods: This retrospective multicenter study, part of the ARON-1 project, aimed to evaluate the outcomes of favorable-risk metastatic RCC patients treated with immune-based combinations or sunitinib. Patients were assessed for overall survival (OS), progression-free survival (PFS) and overall response rate. We carried out a survival analysis by a Cox regression model.
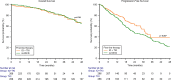
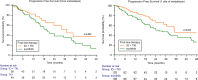
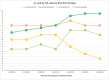
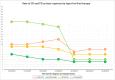
Results: A total of 524 favorable-risk patients were included in the analysis. After a median follow-up of 37.2 months, the median OS in the overall population was 56.1 months. There was no significant difference in OS between patients receiving sunitinib and those receiving TKI + ICI combinations (p = 0.761). Patients on TKI + ICI had significantly longer PFS compared to patient treated with sunitinib (30.7 vs 22.9 months, p = 0.007). Analysis of OS and PFS based on metastatic site revealed that patients with bone metastases benefited more from ICI plus TKI (56 patients with bone metastases receiving IO + TKI, 38 received pembrolizumab plus axitinib, 15 cabozantinib plus nivolumab and 3 pembrolizumab plus lenvatinib), while sunitinib was more effective for pancreatic and glandular metastases. Additionally, the number of metastatic sites played a role, with TKI plus ICI showing superiority in patients with a single metastatic site. The time from RCC diagnosis to metastatic disease also impacted outcomes, with TKI plus ICI being more effective in patients with a shorter interval (i.e., < 36 months).
Conclusions: The choice between upfront combination or monotherapy for metastatic favorable prognosis RCC remains a current issue. While combination therapy offers prolonged PFS, it does not necessarily translate to improve OS compared to sunitinib. This real-world study supports the superiority in terms of PFS of TKI plus ICI vs TKI monotherapy but not in OS. Probable, other clinical factors should be taking into account to make clinical treatment decisions in this setting.
Keywords: ARON-1 study; Good favorable-risk IMDC criteria; Immune-based combinations; Immunotherapy; Renal cell carcinoma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Javier Molina-Cerrillo declares consultant, advisory or speaker roles for IPSEN, Roche, Pfizer, Sanofi, Janssen and BMS. JMC has received research grants from Pfizer, IPSEN and Roche Francesco Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD and Pfizer outside the submitted work. Linda Cerbone has received honoraria for advisory boards, speaker engagements and scientific consultancy for educational purposes from AstraZeneca, EISAI, MSD, Ipsen, BMS, A.A.A.; past MSD employee in Medical Affairs. Ondrej Fiala received honoraria from Novartis, Janssen, Merck and Pfizer for consultations and lectures unrelated to this project. Fernando Sabino M. Monteiro has received research support from Janssen, Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb and Merck Sharp Dome, all unrelated to the present paper. R. Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies; Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas and Bayer. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, Ipsen and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai and MSD. Sebastiano Buti has received honoraria for speaking at scientific events and advisory roles from AstraZeneca, Bristol Myers Squibb, Ipsen, Merck, Eisai, MSD, Novartis and Pfizer and research funding from Novartis and Pfizer. Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas, A.A.A. and Bayer, all unrelated to the present paper. The other authors declare no conflicts of interest.
Figures
Similar articles
-
Real-world short-term outcomes and treatment regimen comparisons in patients with metastatic renal cell carcinoma treated with first-line immune combinations.BMC Cancer. 2025 Jan 22;25(1):117. doi: 10.1186/s12885-025-13504-6. BMC Cancer. 2025. PMID: 39844084 Free PMC article.
-
Targeted therapy for metastatic renal cell carcinoma.Cochrane Database Syst Rev. 2020 Oct 14;10(10):CD012796. doi: 10.1002/14651858.CD012796.pub2. Cochrane Database Syst Rev. 2020. PMID: 33058158 Free PMC article.
-
Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study.Lancet Oncol. 2021 Jul;22(7):946-958. doi: 10.1016/S1470-2045(21)00241-2. Epub 2021 Jun 15. Lancet Oncol. 2021. PMID: 34143969 Free PMC article. Clinical Trial.
-
Combination antiangiogenic tyrosine kinase inhibition and anti-PD1 immunotherapy in metastatic renal cell carcinoma: A retrospective analysis of safety, tolerance, and clinical outcomes.Cancer Med. 2021 Apr;10(7):2341-2349. doi: 10.1002/cam4.3812. Epub 2021 Mar 1. Cancer Med. 2021. PMID: 33650321 Free PMC article.
-
Efficacy of VEGFR-TKIs plus immune checkpoint inhibitors in metastatic renal cell carcinoma patients with favorable IMDC prognosis.Cancer Treat Rev. 2021 Nov;100:102295. doi: 10.1016/j.ctrv.2021.102295. Epub 2021 Sep 20. Cancer Treat Rev. 2021. PMID: 34564043 Review.
Cited by
-
Anti-Cancer Drugs: Trends and Insights from PubMed Records.Pharmaceutics. 2025 May 4;17(5):610. doi: 10.3390/pharmaceutics17050610. Pharmaceutics. 2025. PMID: 40430901 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48. 10.3322/caac.21763 - PubMed
-
- Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127. 10.1056/NEJMoa1816714 - PubMed
-
- Motzer R, Alekseev B, Rha SY et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300. 10.1056/NEJMoa2035716 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

