PSMA-PET/CT Findings in Patients With High-Risk Biochemically Recurrent Prostate Cancer With No Metastatic Disease by Conventional Imaging
- PMID: 39752157
- PMCID: PMC11699533
- DOI: 10.1001/jamanetworkopen.2024.52971
PSMA-PET/CT Findings in Patients With High-Risk Biochemically Recurrent Prostate Cancer With No Metastatic Disease by Conventional Imaging
Abstract
Importance: The phase 3 randomized EMBARK trial evaluated enzalutamide with or without leuprolide in high-risk nonmetastatic hormone-sensitive prostate cancer. Eligibility relied on conventional imaging, which underdetects metastatic disease compared with prostate-specific membrane antigen-positron emission tomography (PSMA-PET).
Objective: To describe the staging information obtained by PSMA-PET/computed tomography (PSMA-PET/CT) in a patient cohort eligible for the EMBARK trial.
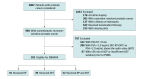
Design, setting, and participants: This post hoc, retrospective cross-sectional study included 182 patients from 4 prospective studies conducted from September 15, 2016, to September 27, 2021. All patients had recurrent prostate cancer after radical prostatectomy (RP), definitive radiotherapy (dRT), or salvage radiotherapy (SRT). Analysis was performed from January 2023 to July 2024.
Exposures: Patients included had increasing prostate-specific antigen (PSA) levels greater than 1.0 ng/mL (after RP and SRT) or 2.0 ng/mL above the nadir value (after dRT), PSA doubling time of 9 months or less, and a serum testosterone level of 150 ng/dL or greater. Exclusion criteria were distant metastatic disease on radiographic imaging and prior hormonal or systemic therapy.
Main outcomes and measures: Staging information obtained by PSMA-PET/CT in patients with nonmetastatic disease according to conventional imaging.
Results: From 2002 patients screened, 182 (median age at PET/CT scan, 69 years [IQR, 64-73 years]) were included. Median prescan PSA levels were 2.4 ng/mL (IQR, 1.4-4.8 ng/mL) after RP (n = 91), 6.9 ng/mL (IQR, 3.5-18.5 ng/mL) after dRT (n = 39), 2.6 ng/mL (IQR, 1.6-5.2 ng/mL) after RP and SRT (n = 52), and 2.8 ng/mL (IQR, 1.7-6.6 ng/mL) overall (n = 182). Results of PSMA-PET were positive in 80% of patients (73 of 91) after RP, 92% of patients (36 of 39) after dRT, 85% of patients (44 of 52) after RP and SRT, and 84% of patients (153 of 182) overall. PSMA-PET detected any distant metastatic disease (miTxNxM1) in 34% of patients (31 of 91) after RP, 56% of patients (22 of 39) after dRT, 60% of patients (31 of 52) after RP and SRT, and 46% of patients (84 of 182) overall. Polymetastatic disease (≥5 lesions) was found in 19% of patients (17 of 91) after RP, 36% of patients (14 of 39) after dRT, 23% of patients (12 of 52) after RP and SRT, and 24% of patients (43 of 182) overall.
Conclusions and relevance: In a cohort of patients with high-risk hormone-sensitive prostate cancer without evidence of metastatic disease by conventional imaging, PSMA-PET results were positive in 84% of patients, detected M1 disease stage in 46% of patients, and found polymetastatic disease (≥5 lesions) in 24% of patients, suggesting that patients' high-risk nonmetastatic hormone-sensitive prostate cancers are understaged by conventional imaging. The results challenge the interpretation of previous studies, such as the EMBARK trial, and support the evolving role of PSMA-PET for patient selection in clinical and trial interventions in prostate cancer. Further studies are needed to assess its independent prognostic value and use for treatment guidance.
Conflict of interest statement
Figures

Comment in
-
Beyond the AJR: When Nonmetastatic Disease Is No Longer Nonmetastatic.AJR Am J Roentgenol. 2025 Nov;225(5):e2532975. doi: 10.2214/AJR.25.32975. Epub 2025 Mar 26. AJR Am J Roentgenol. 2025. PMID: 40135838 No abstract available.
References
-
- Freedland SJ, De Giorgi U, Gleave M, et al. A phase 3 randomised study of enzalutamide plus leuprolide and enzalutamide monotherapy in high-risk non-metastatic hormone-sensitive prostate cancer with rising PSA after local therapy: EMBARK study design. BMJ Open. 2021;11(8):e046588. doi: 10.1136/bmjopen-2020-046588 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

