Site-Selective Copper(I)-Catalyzed Hydrogenation of Amides
- PMID: 39752259
- PMCID: PMC11744755
- DOI: 10.1021/jacs.4c14174
Site-Selective Copper(I)-Catalyzed Hydrogenation of Amides
Abstract
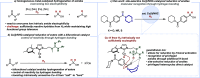
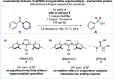
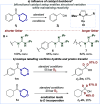
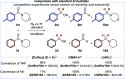
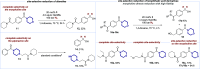
We present a bifunctional catalyst consisting of a copper(I)/N-heterocyclic carbene and an organocatalytic guanidine moiety that enables, for the first time, a copper(I)-catalyzed reduction of amides with H2 as the terminal reducing agent. The guanidine allows for reactivity tuning of the originally weakly nucleophilic copper(I) hydrides - formed in situ - to be able to react with difficult-to-reduce amides. Additionally, the guanidine moiety is key for the selective recognition of "privileged" amides based on simple and readily available heterocycles in the presence of other amides within one molecule, giving rise to hitherto unknown site-selective catalytic amide hydrogenation. A substrate scope, mechanistic investigations, and a working hypothesis supported by computational analysis for site-selectivity are presented.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
LinkOut - more resources
Full Text Sources

