Intracerebroventricular administration of a modified hexosaminidase ameliorates late-stage neurodegeneration in a GM2 mouse model
- PMID: 39752451
- PMCID: PMC11698352
- DOI: 10.1371/journal.pone.0315005
Intracerebroventricular administration of a modified hexosaminidase ameliorates late-stage neurodegeneration in a GM2 mouse model
Abstract
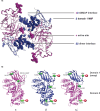
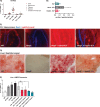
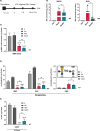
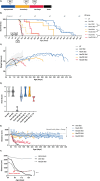
The GM2 gangliosidoses, Tay-Sachs disease and Sandhoff disease, are devastating neurodegenerative disorders caused by β-hexosaminidase A (HexA) deficiency. In the Sandhoff disease mouse model, rescue potential was severely reduced when HexA was introduced after disease onset. Here, we assess the effect of recombinant HexA and HexD3, a newly engineered mimetic of HexA optimized for the treatment of Tay-Sachs disease and Sandhoff disease. Enzyme replacement therapy was administered by repeat intracerebroventricular injections in Sandhoff disease model mice with dosing beginning before and after signs of neurodegeneration. As previously observed, HexA effectively increased the lifespan of Sandhoff disease mice by 3.5-fold only when treatment was started before onset of neurodegeneration. In contrast, HexD3 halted motor decline and ameliorated late-stage disease severity even when dosing began late, after neurodegeneration onset. Additionally, HexD3 had advantages over HexA in enzyme stability, distribution potential, and homodimer activity. Overall, our data indicate that advanced therapeutics may widen the treatment window for neurodegenerative disorders.
Copyright: © 2025 Lopez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
All authors are employees of BioMarin Pharmaceutical, Inc. or were employees at time work was completed. D.W., M.A-S., R.L., and M.L. are inventors on patent application # 63/007,260 entitled “Variants of Hexosaminidase and Uses Thereof” submitted by BioMarin Pharmaceutical, Inc. that covers use of HexD3 to treat Tay-Sachs or Sandhoff disease. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis.Ann Neurol. 2011 Apr;69(4):691-701. doi: 10.1002/ana.22262. Epub 2010 Dec 8. Ann Neurol. 2011. PMID: 21520232
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
-
Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases.Hum Mol Genet. 1997 Oct;6(11):1879-85. doi: 10.1093/hmg/6.11.1879. Hum Mol Genet. 1997. PMID: 9302266
-
Therapeutic potential of intracerebroventricular replacement of modified human β-hexosaminidase B for GM2 gangliosidosis.Mol Ther. 2011 Jun;19(6):1017-24. doi: 10.1038/mt.2011.27. Epub 2011 Apr 12. Mol Ther. 2011. PMID: 21487393 Free PMC article.
-
The biochemistry of HEXA and HEXB gene mutations causing GM2 gangliosidosis.Biochim Biophys Acta. 1991 Feb 22;1096(2):87-94. doi: 10.1016/0925-4439(91)90044-a. Biochim Biophys Acta. 1991. PMID: 1825792 Review. No abstract available.
References
-
- Lyn N, Pulikottil-Jacob R, Rochmann C, Krupnick R, Gwaltney C, Stephens N, et al.. Patient and caregiver perspectives on burden of disease manifestations in late-onset Tay-Sachs and Sandhoff diseases. Orphanet J Rare Dis. 2020;15(1):92. doi: 10.1186/s13023-020-01354-3 ; PubMed Central PMCID: PMC7160997. - DOI - PMC - PubMed
-
- Maegawa GH, Stockley T, Tropak M, Banwell B, Blaser S, Kok F, et al.. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics. 2006;118(5):e1550–62. doi: 10.1542/peds.2006-0588 ; PubMed Central PMCID: PMC2910078. - DOI - PMC - PubMed
-
- Tropak MB, Yonekawa S, Karumuthil-Melethil S, Thompson P, Wakarchuk W, Gray SJ, et al.. Construction of a hybrid beta-hexosaminidase subunit capable of forming stable homodimers that hydrolyze GM2 ganglioside in vivo. Mol Ther Methods Clin Dev. 2016;3:15057. doi: 10.1038/mtm.2015.57 ; PubMed Central PMCID: PMC4774620. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

