Large-scale multicenter study reveals anticitrullinated SR-A peptide antibody as a biomarker and exacerbator for rheumatoid arthritis
- PMID: 39752500
- PMCID: PMC11698088
- DOI: 10.1126/sciadv.adr8078
Large-scale multicenter study reveals anticitrullinated SR-A peptide antibody as a biomarker and exacerbator for rheumatoid arthritis
Abstract
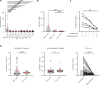
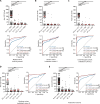
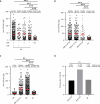
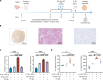
Current diagnosis and treatment of rheumatoid arthritis (RA) is still challenging. More than one-third of patients with RA could not be accurately diagnosed because of lacking biomarkers. Our recent study reported that scavenger receptor-A (SR-A) is a biomarker for RA, especially for anticyclic citrullinated peptide antibody (anti-CCP)-negative RA. Here, we further identified the B cell autoantigenic epitopes of SR-A. By a large-scale multicenter study including one training and three validation cohorts of 1954 participants, we showed that anticitrullinated SR-A peptide antibody (anti-CSP) was exclusively elevated in RA as a biomarker, particularly useful for seronegative RA. Combination of anti-CSP with anti-CCP demonstrated superior diagnostic value for RA, with sensitivity of 84.83% and specificity of 92.43%. Moreover, RA anti-CSP revealed distinct glycosylation patterns, capable of provoking inflammation in cartilage organoids and exacerbating disease progression in experimental arthritis. Together, these data identify anti-CSP as an RA autoantibody clinically applicable and actively involved in disease pathogenesis.
Figures


References
-
- Smolen J. S., Aletaha D., McInnes I. B., Rheumatoid arthritis. Lancet 388, 2023–2038 (2016). - PubMed
-
- Ahsan H., Origins and history of autoimmunity—A brief review. Rheumatol. Autoimmun. 3, 9–14 (2023).
-
- Van Hoovels L., Jacobs J., Vander Cruyssen B., Van den Bremt S., Verschueren P., Bossuyt X., Performance characteristics of rheumatoid factor and anti-cyclic citrullinated peptide antibody assays may impact ACR/EULAR classification of rheumatoid arthritis. Ann. Rheum. Dis. 77, 667–677 (2018). - PubMed
-
- Nishimura K., Sugiyama D., Kogata Y., Tsuji G., Nakazawa T., Kawano S., Saigo K., Morinobu A., Koshiba M., Kuntz K. M., Kamae I., Kumagai S., Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann. Intern. Med. 146, 797–808 (2007). - PubMed
-
- Curran A. M., Naik P., Giles J. T., Darrah E., PAD enzymes in rheumatoid arthritis: Pathogenic effectors and autoimmune targets. Nat. Rev. Rheumatol. 16, 301–315 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

