A propofol binding site in the voltage sensor domain mediates inhibition of HCN1 channel activity
- PMID: 39752505
- PMCID: PMC11698089
- DOI: 10.1126/sciadv.adr7427
A propofol binding site in the voltage sensor domain mediates inhibition of HCN1 channel activity
Abstract
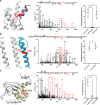
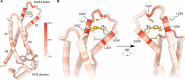
Hyperpolarization-activated and cyclic nucleotide-gated (HCN) ion channels are members of the cyclic nucleotide-binding family and are crucial for regulating cellular automaticity in many excitable cells. HCN channel activation contributes to pain perception, and propofol, a widely used anesthetic, acts as an analgesic by inhibiting the voltage-dependent activity of HCN channels. However, the molecular determinants of propofol action on HCN channels remain unknown. Here, we use a propofol-analog photoaffinity labeling reagent to identify propofol binding sites in the human HCN1 isoform. Mass spectrometry analyses combined with molecular dynamics simulations show that a binding pocket is formed by extracellularly facing residues in the S3 and S4 transmembrane segments in the resting voltage-sensor conformation. Mutations of residues within the putative binding pocket mitigate or eliminate voltage-dependent modulation of HCN1 currents by propofol. Together, these findings reveal a conformation-specific propofol binding site that underlies voltage-dependent inhibition of HCN currents and provides a framework for identifying highly specific modulators of HCN channel gating.
Figures


References
-
- Biel M., Wahl-Schott C., Michalakis S., Zong X., Hyperpolarization-activated cation channels: From genes to function. Phys. Rev. 89, 847–885 (2009). - PubMed
-
- Craven K., Zagotta W. N., CNG and HCN channels: Two peas, one pod. Annu. Rev. Physiol. 68, 374–401 (2006). - PubMed
-
- Santoro B., Liu D. T., Yao H., Bartsch D., Kandel E. R., Siegelbaum S. A., Tibbs G. R., Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93, 717–729 (1998). - PubMed
-
- Brown H. F., Difrancesco D., Noble S. J., How does adrenaline accelerate the heart? Nature 280, 235–236 (1979). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

