Experimental medicine study with stabilised native-like HIV-1 Env immunogens drives long-term antibody responses, but lacks neutralising breadth
- PMID: 39753033
- PMCID: PMC11753977
- DOI: 10.1016/j.ebiom.2024.105544
Experimental medicine study with stabilised native-like HIV-1 Env immunogens drives long-term antibody responses, but lacks neutralising breadth
Abstract
Background: We report findings from an experimental medicine study of rationally designed prefusion stabilised native-like HIV envelope glycoprotein (Env) immunogens, representative of global circulating strains, delivered by sequential intramuscular injection.
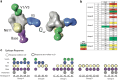
Methods: Healthy adult volunteers were enrolled into one of five groups (A to E) each receiving a different schedule of one of two consensus Env immunogens (ConM SOSIP, ConS UFO, either unmodified or stabilised by chemical cross-linking, followed by a boost with two mosaic Env immunogens (Mos3.1 and Mos3.2). All immunogens were co-formulated with liposomal Monophosphoryl-Lipid A (MPLA) adjuvant, and volunteers were followed up for 28 days post final Mosaic booster injection. Participants gave written informed consent to join the study. The study is registered on ClinicalTrials.gov ID NCT03816137.
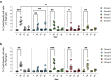
Findings: Fifty-one participants (men n = 23 and women n = 28) aged 18-55 were enrolled. The seroconversion rate against Env was 100% with all participants having measurable anti-Env IgG antibodies after their second injection and throughout the study. Neutralisation was detected against the ConM pseudovirus in sera of those who had received both ConM and ConS immunogens. However, this activity was limited in breadth and was neither boosted nor broadened in those receiving the Mos3.1 and Mos3.2 immunogens. Neutralising antibody function correlated with binding to V1/V3 and V5 epitopes and peaked after the third injection.
Interpretation: Rationally designed prefusion-stabilised native-like Env trimers are robustly immunogenic in a prime-boost schedule. When given alone they are insufficient to induce neutralising antibody titres of significant breadth, but they represent potentially valuable polishing immunogens after germline-targeting.
Funding: European Aids Vaccine initiative (EAVI2020) received funding from EU Horizon 2020, grant number 681137. Structural studies were supported by the Bill and Melinda Gates Foundation (INV-002916).
Keywords: Experimental medicine; HIV envelope; HIV-1; Prefusion; Trimer; Vaccine.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests K.M.P was supported in part by the NIHR Imperial Biomedical Research Centre and by the St Mary’s Development Trust, and has received research funding support from Horizon 2020 and from Trevena Inc, Imperial COVID-19 fund, National Institute for Health Research, and The Sir Joseph Hotung Charitable Settlement outside the submitted work. K.M.P is in receipt of an MRC Clinician Scientist Fellowship award (MR/W024977/1) and a grant from the Chan Zuckerberg Initiative outside the submitted work. K.M.P has received payment or honoraria from CSL Seqirus and Sanofi Pasteur for speaking and as a panellist, and travel support from the Chan Zuckerberg Initiative. K.M.P has participated in data safety monitoring boards for Moderna. K.M.P has a role on the British HIV Association immunisation guidelines writing committee and was on the UK Chief Investigators Group NIHR vaccine research programme. R.W.S and I.B declare grants or contracts from any entity - EU (EAVI2020; grant number 681137). B.K. is a co-inventor on a patent covering modified human immunodeficiency virus type 1 (HIV-1) group M consensus envelope glycoproteins (Mosaic) [US-9844589-B2]. A.B.W declares that this work was supported, in whole or in part, by the Bill & Melinda Gates Foundation INV-002916. All other authors declare no conflict of interests regarding any financial and personal relationships with other people or organisations that could inappropriately influence our work.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

