Classification of non-TCGA cancer samples to TCGA molecular subtypes using compact feature sets
- PMID: 39753139
- PMCID: PMC11949768
- DOI: 10.1016/j.ccell.2024.12.002
Classification of non-TCGA cancer samples to TCGA molecular subtypes using compact feature sets
Abstract
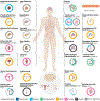
Molecular subtypes, such as defined by The Cancer Genome Atlas (TCGA), delineate a cancer's underlying biology, bringing hope to inform a patient's prognosis and treatment plan. However, most approaches used in the discovery of subtypes are not suitable for assigning subtype labels to new cancer specimens from other studies or clinical trials. Here, we address this barrier by applying five different machine learning approaches to multi-omic data from 8,791 TCGA tumor samples comprising 106 subtypes from 26 different cancer cohorts to build models based upon small numbers of features that can classify new samples into previously defined TCGA molecular subtypes-a step toward molecular subtype application in the clinic. We validate select classifiers using external datasets. Predictive performance and classifier-selected features yield insight into the different machine-learning approaches and genomic data platforms. For each cancer and data type we provide containerized versions of the top-performing models as a public resource.
Keywords: TCGA; artificial intelligence; biomarkers; cancer; classification; epigenomic; genomic; machine learning; molecular; pathology.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.D.C. receives research support from Bayer and consults for KaryoVerse. W.R. is currently working at Pfizer. I.T., P.C., and V.L. are or were directly or indirectly affiliated with JADBio—Gnosis DA, S.A., which offers the JADBio service commercially. V.F. is an employee and stock option owner of Bluestar Genomics Inc. L.R.R. has received grants from Bayer, Boston Scientific, Exact Sciences, FujiFilm Medical Sciences, Gilead Sciences, GlycoTest, RedHill, and Target PharmaSolutions and serves in a consulting/advising role for AstraZeneca, Bayer, Eisai, Exact Sciences, Gilead Sciences, Global Life Science Consulting, GRAIL, LLC, Hepion, MedEd Design, Medscape, Novartis Venture Fund, QED, RedHill, and The Lynx Group. A.J.L. has consulting relationships with AbbVie, Astra-Zeneca, Bayer, Bio-AI Health, BMS, Caris, Deciphera, Foghorn Therapeutics, GRAIL, GSK, Illumina, Invitae/Archer DX, Iterion Therapeutics, Merck, Novartis, Nucleai, Paige, Pfizer, Regeneron, Roche/Genentech, SpringWorks, Tempus, and ThermoFisher. W.R. and G.G. are co-inventors of a patent application related to lung adenocarcinoma expression subtypes (U.S. Provisional Patent Application No.: 63/293,349). P.W.L. serves on the Scientific Advisory Boards of FOXO Technologies, Inc., and Tagomics, LLC. J.M.S. is a stock owner of Nantomics Inc. V.U. is an employee and stock owner of Bristol Myers Squibb.
Figures






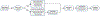
References
-
- Kumar V, Abbas AK, and Aster JC (2021). Robbins & Cotran Pathologic Basis of Disease, 10th Edition (Elsevier; ), pp. 1–1392.
-
- Sobin LH, Gospodarowicz MK, and Wittekind C (2011). TNM Classification of Malignant Tumours, 7th Edition (John Wiley & Sons; ), pp. 1–336.

