Targeted metabolomics reveals novel diagnostic biomarkers for colorectal cancer
- PMID: 39753208
- PMCID: PMC12161463
- DOI: 10.1002/1878-0261.13791
Targeted metabolomics reveals novel diagnostic biomarkers for colorectal cancer
Abstract
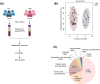
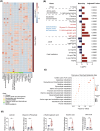
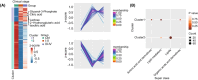
Colorectal cancer (CRC) is a prevalent malignant tumor worldwide, with a high mortality rate due to its complex etiology and limited early screening techniques. This study aimed to identify potential biomarkers for early detection of CRC utilizing targeted metabolite profiling of platelet-rich plasma (PRP). Based on multiple reaction monitoring (MRM) mode, liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis identified metabolites in PRP collected from patients with CRC (n = 70) and healthy controls (n = 30). A total of 302 metabolites were identified and quantified in this study, including various categories such as lipids, lipid mediators, amino acids, and derivatives, organic acids and derivatives, nucleotides and derivatives, alkaloids, carbohydrates, vitamins and derivatives, and others. The differential analysis revealed that five carbohydrates and organic acids (lactose, glycerol-3-phosphate, 2-hydroxyglutaric acid, isocitric acid, and citric acid) involved in the carbohydrate metabolism pathway displayed consistent upregulation within PRP derived from patients with CRC. To further validate the abundance of differential metabolites, 10 pairs of CRC tissues, adjacent tissues, and matched PRP were collected. Ultimately, five carbohydrate metabolites were validated in PRP, and compared with carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA199), the five carbohydrate metabolites significantly improved the specificity of differentiating patients with CRC from healthy controls. Furthermore, the diagnostic efficacy of the combined five-carbohydrate metabolite panel was superior to that of individual metabolites, CEA and CA199. The sensitivity, specificity, and AUC of the metabolite panel in distinguishing patients with CRC from healthy controls were 90.00%, 96.67%, and 0.961 (95% CI 0.922-0.998), respectively. Collectively, metabolomics was used to identify and validate differential metabolites in the PRP of CRC, which may serve as potential early screening markers for patients with CRC.
Keywords: carbohydrate metabolites; colorectal cancer; diagnostic model; metabolomics; platelet rich plasma.
© 2025 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Bagheri R, Ghorbian M, Ghorbian S. Tumor circulating biomarkers in colorectal cancer. Cancer Treat Res Commun. 2024;38:100787. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

