Localized In Vivo Prodrug Activation Using Radionuclides
- PMID: 39753366
- PMCID: PMC11705795
- DOI: 10.2967/jnumed.124.268559
Localized In Vivo Prodrug Activation Using Radionuclides
Abstract
Radionuclides used for imaging and therapy can show high molecular specificity in the body with appropriate targeting ligands. We hypothesized that local energy delivered by molecularly targeted radionuclides could chemically activate prodrugs at disease sites while avoiding activation in off-target sites of toxicity. As proof of principle, we tested whether this strategy of radionuclide-induced drug engagement for release (RAiDER) could locally deliver combined radiation and chemotherapy to maximize tumor cytotoxicity while minimizing off-target exposure to activated chemotherapy. Methods: We screened the ability of radionuclides to chemically activate a model radiation-activated prodrug consisting of the microtubule-destabilizing monomethyl auristatin E (MMAE) caged by a radiation-responsive phenyl azide, and we interpreted experimental results using the radiobiology computational simulation suite TOPAS-nBio. RAiDER was evaluated in syngeneic mouse models of cancer using the fibroblast activation protein inhibitor (FAPI) agents [99mTc]Tc-FAPI-34 and [177Lu]Lu-FAPI-04 and the prostate-specific membrane antigen (PSMA) agent [177Lu]Lu-PSMA-617, combined with caged MMAE or caged exatecan. Biodistribution in mice, combined with clinical dosimetry, estimated the relationship between radiopharmaceutical uptake in patients and anticipated concentrations of activated prodrug using RAiDER. Results: RAiDER efficiency varied by 70-fold across radionuclides (99mTc > 111In > 177Lu > 64Cu > 32P > 68Ga > 223Ra > 18F), yielding up to 320 nM prodrug activation/Gy of exposure from 99mTc. Computational simulations implicated low-energy electron-mediated free radical formation as driving prodrug activation. Radionuclide-activated caged MMAE restored the prodrug's ability to destabilize microtubules and increased its cytotoxicity by up to 2,600-fold that of the nonactivated prodrug. Mice treated with [99mTc]Tc-FAPI-34 and caged MMAE accumulated concentrations of activated MMAE that were up to 3,000 times greater in tumors than in other tissues. RAiDER with [99mTc]Tc-FAPI-34 or [177Lu]Lu-FAPI-04 delayed tumor growth, whereas monotherapies did not (P < 0.003). Clinically guided dosimetry suggests sufficient radiation doses can be delivered to activate therapeutically meaningful levels of prodrug. Conclusion: This proof-of-concept study shows that RAiDER is compatible with multiple radionuclides commonly used in nuclear medicine and can potentially improve the efficacy of radiopharmaceutical therapies to treat cancer safely. RAiDER thus shows promise as an effective strategy to treat disseminated malignancies and broadens the capability of radiopharmaceuticals to trigger diverse biologic and therapeutic responses.
Keywords: FAPI; combination chemoradiotherapy; radionuclide therapy; theranostics.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
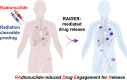



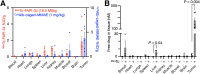
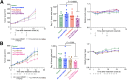
Update of
-
Localized in vivo prodrug activation using radionuclides.bioRxiv [Preprint]. 2024 Aug 6:2024.08.02.606075. doi: 10.1101/2024.08.02.606075. bioRxiv. 2024. Update in: J Nucl Med. 2025 Jan 3;66(1):91-97. doi: 10.2967/jnumed.124.268559. PMID: 39211146 Free PMC article. Updated. Preprint.
References
-
- Strosberg JR, Wolin EM, Chasen B, et al. NETTER-1 phase III: progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177Lu-DOTATATE [abstract]. J Clin Oncol. 2016;34(suppl):194.
-
- Dagher OK, Schwab RD, Brookens SK, Posey AD. Advances in cancer immunotherapies. Cell. 2023;186:1814–1814.e1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
