Macrophage STING signaling promotes fibrosis in benign airway stenosis via an IL6-STAT3 pathway
- PMID: 39753529
- PMCID: PMC11698984
- DOI: 10.1038/s41467-024-55170-5
Macrophage STING signaling promotes fibrosis in benign airway stenosis via an IL6-STAT3 pathway
Abstract
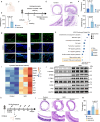
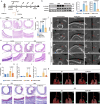
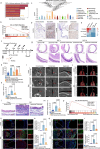
Acute and chronic inflammation are important pathologies of benign airway stenosis (BAS) fibrosis, which is a frequent complication of critically ill patients. cGAS-STING signalling has an important role in inflammation and fibrosis, yet the function of STING in BAS remains unclear. Here we demonstrate using scRNA sequencing that cGAS‒STING signalling is involved in BAS, which is accompanied by increased dsDNA, expression and activation of STING. STING inhibition or deficiency effectively alleviates tracheal fibrosis of BAS mice by decreasing both acute and chronic inflammation. Macrophage depletion also effectively ameliorates BAS. Mechanistically, dsDNA from damaged epithelial cells activates the cGAS-STING pathway of macrophages and induces IL-6 to activate STAT3 and promote fibrosis. In summary, the present results suggest that cGAS-STING signalling induces acute inflammation and amplifies the chronic inflammation and tracheal fibrosis associated with benign airway stenosis, highlighting the mechanism and potential drug target of BAS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- SRA/SRR31229501
- SRA/SRR31229502
- SRA/SRR31229503
- SRA/SRR31229504
- SRA/SRR31229505
- SRA/SRR31229506
- SRA/SRR31229507
- SRA/SRR30898181
- SRA/SRR30898182
- SRA/SRR30898183
- SRA/SRR30898184
- SRA/SRR30898185
- SRA/SRR30898186
- SRA/SRR30898187
- SRA/SRR30898188
- SRA/SRR30898189
- SRA/SRR30831591
- SRA/SRR30831592
- SRA/SRR30831593
- SRA/SRR30831594
- SRA/SRR30831595
- SRA/SRR30831596
- SRA/SRR30831597
- SRA/SRR30831598
- SRA/SRR30831590
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

