Therapy-induced senescent cancer cells contribute to cancer progression by promoting ribophorin 1-dependent PD-L1 upregulation
- PMID: 39753537
- PMCID: PMC11699195
- DOI: 10.1038/s41467-024-54132-1
Therapy-induced senescent cancer cells contribute to cancer progression by promoting ribophorin 1-dependent PD-L1 upregulation
Abstract
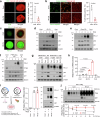
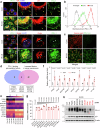
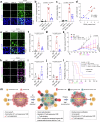
Conventional chemotherapy- and radiotherapy-induced cancer senescence, which is characterized by poor proliferation, drug resistance, and senescence-associated secretory phenotype, has gained attention as contributing to cancer relapse and the development of an immunosuppressive tumor microenvironment. However, the association between cancer senescence and anti-tumor immunity is not fully understood. Here, we demonstrate that senescent cancer cells increase the level of PD-L1 by promoting its transcription and glycosylation. We identify ribophorin 1 as a key regulator of PD-L1 glycosylation during cancer senescence. Ribophorin 1 depletion reduces this elevated level of PD-L1 through the ER-lysosome-associated degradation pathway, thereby increasing the susceptibility of senescent cancer cells to T-cell-mediated killing. Consistently, ribophorin 1 depletion suppresses tumor growth by decreasing PD-L1 levels and boosting cytotoxic T lymphocyte activity in male mice. Moreover, ribophorin 1-targeted or anti-PD-1 therapy reduces the number of senescent cancer cells in irradiated tumors and suppresses cancer recurrence through the activation of cytotoxic T lymphocytes. These results provide crucial insights into how senescent cancer cells can escape T-cell immunity following cancer treatment and thereby contribute to cancer recurrence. Our findings also highlight the therapeutic promise of targeting senescent cancer cells for cancer treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

