Tandospirone prevents anesthetic-induced respiratory depression through 5-HT1A receptor activation in rats
- PMID: 39753642
- PMCID: PMC11698729
- DOI: 10.1038/s41598-024-84440-x
Tandospirone prevents anesthetic-induced respiratory depression through 5-HT1A receptor activation in rats
Abstract
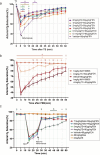
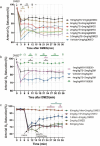
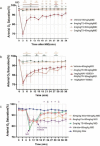
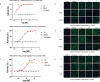
Respiratory depression is a side effect of anesthetics. Treatment with specific antagonists or respiratory stimulants can reverse respiratory depression caused by anesthetics; however, they also interfere with the sedative effects of anesthetics. Previous studies have suggested that tandospirone may ameliorate respiratory depression without affecting the sedative effects of anesthetics. Therefore, we evaluated whether tandospirone (0.1-8 mg/kg) ameliorates respiratory depression in a rat model under anesthesia. The protein kinase A redistribution method was used to determine whether tandospirone activates α2a/2c and µ receptors. The effects of tandospirone (10 µM) on α1β2γ2 and α4β2δ GABA receptor current modulation were explored by two-electrode voltage clamping. Prophylactic tandospirone administration reduced respiratory depression caused by anesthetics in rats. Tandospirone (0.1-8 mg/kg) increased SaO2 in rats treated with fentanyl (80 µg/kg) or midazolam (80 mg/kg) (P < 0.05). The ability of tandospirone to prevent respiratory depression was inhibited by the 5-hydroxytryptamine (5-HT)1 A receptor antagonist WAY100635 (1 mg/kg) (P < 0.05). Co-administration of tandospirone with dexmedetomidine or fentanyl did not affect α2a/2c or µ receptors activation. Tandospirone (10 µM) did not affect α1β2γ2 and α4β2δ GABA receptor modulation (P < 0.05). Overall, tandospirone ameliorated respiratory depression caused by anesthetics in rats through 5-HT1A receptor activation.
Keywords: 5-HT1A; Dexmedetomidine; Fentanyl; Midazolam; Respiratory depression; Tandospirone.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: We confirm that all experiments were performed in accordance with relevant named guidelines and regulations, and that the authors complied with the ARRIVE guidelines.All animal housing and experiments were conducted in strict accordance with the institutional guidelines for care and use of laboratory animals. All experiments were approved by the Institutional Animal Care and Use Committee (approval number: IACUC-DWZX-2023P562).
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

