hsa-mir-483-3p modulates delayed breast cancer recurrence
- PMID: 39753688
- PMCID: PMC11698896
- DOI: 10.1038/s41598-024-84437-6
hsa-mir-483-3p modulates delayed breast cancer recurrence
Erratum in
-
Publisher Correction: hsa-mir-483-3p modulates delayed breast cancer recurrence.Sci Rep. 2025 Apr 8;15(1):12030. doi: 10.1038/s41598-025-95188-3. Sci Rep. 2025. PMID: 40199922 Free PMC article. No abstract available.
Abstract
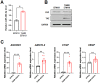
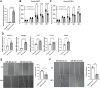
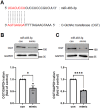
Patients with estrogen receptor-positive breast cancer undergoing continuous adjuvant hormone therapy often experience delayed recurrence with tamoxifen use, potentially causing adverse effects. However, the lack of biomarkers hampers patient selection for extended endocrine therapy. This study aimed to elucidate the molecular mechanisms underlying delayed recurrence and identify biomarkers. When miRNA expression was assessed in luminal breast cancer tissues with and without delayed recurrence using NanoString, a significant increase in the expression of miR483-3p was observed in samples from patients with delayed recurrence compared with those without. miR483-3p expression was elevated in tamoxifen resistant (TAMR) EFM19 cells than in non-resistant EFM19 cells. Notably, genes associated with cancer metastasis (AMOTL2, ANKRD1, CTGF, and VEGF) were upregulated in TAMR EFM19 cells, although cell motility and proliferation were reduced. Transfection of miR483-3p mimics into both non-resistant EFM19 and MCF7 cells resulted in increased expression of cancer metastasis-related genes, but decreased proliferation and migration. Given that miR483-3p can bind to the 3'UTR region of O-GlcNAc transferase (OGT) and potentially affect its protein expression, we examined OGT protein levels and found that transfection with miR483-3p mimics selectively reduced OGT expression. Overall, breast cancer cells subjected to long-term hormone therapy displayed elevated miR483-3p expression, reducing motility and dormancy induction via decreased OGT expression. These findings suggest that miR483-3p is a potential biomarker for long-term endocrine therapy.
Keywords: Breast cancer; Delayed recurrence; Dormancy; miR483-3p.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: For human samples, approval was obtained from the Institutional Review Board of Chung-Ang University Hospital (IRB number 2301-019-539). A waiver of informed consent was granted by the Institutional Review Board of Chung-Ang University Hospital.
Figures


References
-
- Ribnikar, D., Sousa, B., Cufer, T. & Cardoso, F. Extended adjuvant endocrine therapy - A standard to all or some? Breast32, 112–118 (2017). - PubMed
-
- Park, Y. H. et al. Pan-asian adapted ESMO Clinical Practice guidelines for the management of patients with early breast cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol.31, 451–469 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

