Development of the membrane ceiling method for in vitro spermatogenesis
- PMID: 39753886
- PMCID: PMC11699200
- DOI: 10.1038/s41598-024-84965-1
Development of the membrane ceiling method for in vitro spermatogenesis
Abstract
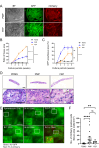
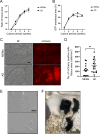
Spermatogenesis is one of the most complex processes of cell differentiation and its failure is a major cause of male infertility. Therefore, a proper model that recapitulates spermatogenesis in vitro has been long sought out for basic and clinical research. Testis organ culture using the gas-liquid interphase method has been shown to support spermatogenesis in mice and rats. However, the conventional method using agarose gel has limitations including medium replacement efficiency and live imaging because agarose absorbs medium and is not transparent. To overcome this issue, we developed a new device using microporous membranes and oxygen-permeable materials. Mouse testes sandwiched between a microporous polyethylene terephthalate (PET) membrane on top and an oxygen-permeable 4-polymethyl-1-pentene polymer (PMP) membrane base maintained spermatogenesis over months. The chamber volume was minimized to 0.1% of the culture medium. Weekly time-lapse live imaging enabled us to observe transgenically fluorescent acrosome and nuclear shape formation throughout spermatogenesis. Finally, we obtained healthy fertile offspring from spermatozoa generated in our system. The device could be used not only for basic research to understand spermatogenesis but also for applied research, such as diagnosing and treating male infertility.
Keywords: 4-Polymethyl-1-pentene polymer (PMP); Fluorinated ethylene-propylene copolymer (FEP); In vitro spermatogenesis; Membrane ceiling chip; Polydimethylsiloxane (PDMS); Testis culture.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Authors K.E., J.Y., and S.Y. were employed by Mitsui Chemicals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- CURI, S. M. et al. Asthenozoospermia: analysis of a large population. Arch. Androl.49, 343–349 (2003). - PubMed
-
- Oura, S., Mori, H. & Ikawa, M. Genome editing in mice and its application to the study of spermatogenesis. Gene Genome Ed.3, 100014 (2022).
-
- Yoshida, S., Sukeno, M. & Nabeshima, Y. A. Vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science317, 1722–1726 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

