Effect of in vitro exposure of first-line antiretrovirals on healthy human spermatozoa on kinematics and motility
- PMID: 39753908
- PMCID: PMC12049304
- DOI: 10.1007/s11255-024-04340-x
Effect of in vitro exposure of first-line antiretrovirals on healthy human spermatozoa on kinematics and motility
Abstract
Purpose: Contemporary antiretroviral (ARV) medications are used by millions of men for HIV treatment worldwide. Limited data exist on their direct effect on sperm motility. This pilot study hypothesizes that in vitro exposure to ARVs will reduce sperm kinematic and motility parameter values.
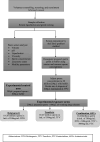
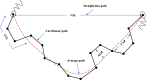
Methods: This laboratory-based experimental study analyzed sperm motility and kinematics after exposure to the ARVs Dolutegravir, Tenofovir, and Emtricitabine, individually and in combination. Each participant (n = 23) served as their experimental control. The Microptic SCA® Computer Assisted Sperm Analysis (CASA) system, Barcelona, Spain was used to generate quantitative data on sperm motility and the kinematics Straight-line velocity (VSL), Straightness index (STR), Linearity Index (LIN), Beat cross frequency (BCF), and the oscillation index (WOB).
Results: VSL, STR, LIN, and WOB of the non-progressive (grade c) spermatozoa were significantly decreased after ARV treatment. BCF of the medium velocity progressive sperm population (grade b) was significantly increased 90 min after exposure in the Tenofovir arm, and a significant decrease in the proportion of grade b spermatozoa was recorded at 90 min in all the antiretroviral arms when compared to the control arm. No impaired sperm motility was observed within the first 30 min of exposure.
Conclusion: Pharmacovigilance is a healthcare emergency as the fast-changing world of newer drugs leaves clinicians vulnerable. They must prescribe drugs whose long-term somatic and germline adverse effects are not fully understood. Guidelines and drugs are changing faster than we can monitor for side effects. Despite Dolutegravir being the only mainstream integrase inhibitor first-line ARV in South Africa for five years, its replacement, Cabotegravir, is already being launched. More research in this field is required, especially for commonly prescribed drugs. This preliminary pilot study concludes that the current first-line ARVs used by HIV patients and HIV-negative patients on pre-exposure prophylaxis (PrEP) can alter sperm motility and kinematics. Further research with a larger sample size is warranted to quantify its impact on human fertility, addressing the limitations of this study, before a comprehensive conclusion of the effects of ARVs on human male fertility can be drawn. Of particular importance would be to study the impact of ARVs on reactive oxygen species levels in semen and sperm DNA fragmentation.
Keywords: Antiretrovirals; Dolutegravir; Emtricitabine; Male infertility; Sperm kinematics; Sperm motility; Tenofovir.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures

References
-
- https://cfs.hivci.org/country-factsheet.html#, accessed Jun 26 2020
-
- Bekker L-G, Rebe K, Venter F, Maartens G, Moorhouse M, Conradie F, Wallis C, Black V, Harley B, Eakle R (2016) Southern African guidelines on the safe use of pre-exposure prophylaxis in persons at risk of acquiring HIV-1 infection. South Afr J HIV Med. 10.4102/sajhivmed.v17i1.455 - DOI - PMC - PubMed
-
- https://www.nicd.ac.za/wp-content/uploads/2019/11/2019-ART-Clinical-Guid..., accessed Sept 24 2020
-
- https://www.who.int/publications/i/item/update-of-recommendations-on-fir..., accessed Jun 26 2020
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

