MTCH2 controls energy demand and expenditure to fuel anabolism during adipogenesis
- PMID: 39753955
- PMCID: PMC11832942
- DOI: 10.1038/s44318-024-00335-7
MTCH2 controls energy demand and expenditure to fuel anabolism during adipogenesis
Abstract
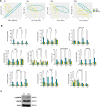
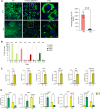
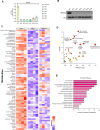
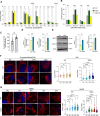
Mitochondrial carrier homolog 2 (MTCH2) is a regulator of apoptosis, mitochondrial dynamics, and metabolism. Loss of MTCH2 results in mitochondrial fragmentation, an increase in whole-body energy utilization, and protection against diet-induced obesity. In this study, we used temporal metabolomics on HeLa cells to show that MTCH2 deletion results in a high ATP demand, an oxidized cellular environment, and elevated utilization of lipids, amino acids, and carbohydrates, accompanied by a decrease in several metabolites. Lipidomics analysis revealed a strategic adaptive reduction in membrane lipids and an increase in storage lipids in MTCH2 knockout cells. Importantly, MTCH2 knockout cells showed an increase in mitochondrial oxidative function, which may explain the higher energy demand. Interestingly, this imbalance in energy metabolism and reductive potential triggered by MTCH2-deletion prevents NIH3T3L1 preadipocytes from differentiating into mature adipocytes, an energy consuming reductive biosynthetic process. In summary, the loss of MTCH2 leads to increased mitochondrial oxidative activity and energy demand, creating a catabolic and oxidative environment that fails to fuel the anabolic processes required for lipid accumulation and adipocyte differentiation.
Keywords: Adipogenesis; Energy Expenditure and Demand; MTCH2; Mitochondrial Oxidative Function.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

