Single immunization with genetically attenuated Pf∆mei2 (GA2) parasites by mosquito bite in controlled human malaria infection: a placebo-controlled randomized trial
- PMID: 39753962
- PMCID: PMC11750698
- DOI: 10.1038/s41591-024-03347-2
Single immunization with genetically attenuated Pf∆mei2 (GA2) parasites by mosquito bite in controlled human malaria infection: a placebo-controlled randomized trial
Abstract
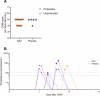
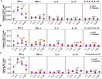
Malaria vaccines consisting of metabolically active Plasmodium falciparum (Pf) sporozoites can offer improved protection compared with currently deployed subunit vaccines. In a previous study, we demonstrated the superior protective efficacy of a three-dose regimen of late-arresting genetically attenuated parasites administered by mosquito bite (GA2-MB) compared with early-arresting counterparts (GA1-MB) against a homologous controlled human malaria infection. Encouraged by these results, we explored the potency of a single GA2-MB immunization in a placebo-controlled randomized trial. Primary outcomes were safety and tolerability, time-to-parasitemia and protective efficacy. Humoral and cellular immunological results were considered secondary outcomes. Here we report the safe administration of GA2-MB with no breakthrough malaria and sterile protection in nine of ten participants at 6 weeks after a single immunization with 50 GA2-infected mosquitoes, compared with none of five mock-immunized participants, against a homologous controlled human malaria infection. Immunization increased circulating Pf-specific polyfunctional effector memory CD4+ T cells coexpressing tumor necrosis factor and interleukin-2. This unprecedented 90% protective efficacy after a single low-dose immunization holds great promise for the potency of GA2 immunization. Future studies should demonstrate whether GA2 is similarly efficacious in pre-exposed populations and whether the favorable safety profile reported here holds up in larger groups. ClinicalTrials.gov registration: NCT05468606 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- World Malaria Report 2022 (World Health Organization, 2022).
-
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) (Institute for Health Metrics and Evaluation, accessed 13 August 2024); https://www.healthdata.org/data-tools-practices/interactive-visuals/gbd-...
-
- Datoo, M. S. et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet403, 533–544 (2024). - PubMed
-
- Malaria Vaccines: Preferred Product Characteristics and Clinical Development Considerations (World Health Organization, 2022).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

