Immobilization of purified pectinase from Aspergillus nidulans on chitosan and alginate beads for biotechnological applications
- PMID: 39754158
- PMCID: PMC11699674
- DOI: 10.1186/s12934-024-02603-x
Immobilization of purified pectinase from Aspergillus nidulans on chitosan and alginate beads for biotechnological applications
Abstract
Background: Because the process is cost-effective, microbial pectinase is used in juice clearing. The isolation, immobilization, and characterization of pectinase from Aspergillus nidulans (Eidam) G. Winter (AUMC No. 7147) were therefore the focus of the current investigation.
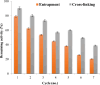
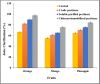
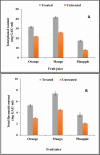
Results: Ammonium sulphate (85%), DEAE-cellulose, and Sephadex G-200 were used to purify the enzyme. With a yield of 30.4%, the final specific activity was 400 units mg-1 protein and 125-fold purification. Using SDS-PAGE to validate the purification of the pectinase, a single band showing the homogeneity of the purified pectinase with a molecular weight of 50 kD was found. Chitosan and calcium alginate both effectively immobilized pectinase, with immobilization efficiencies of 85.7 and 69.4%, respectively. At 50, 55, 60, and 65 °C, the thermostability of both free and chitosan-immobilized pectinase was examined. The free and chitosan-immobilized enzymes had half-lives (t1/2) of 23.83 and 28.64 min at 65 °C, and their Kd values were 0.0291 and 0.0242 min-1, respectively. In addition, the Z values were 44.6 and 31.54 °C, while the D values were 79.2 and 95.1 min. Compared to the untreated one, the orange, mango, and pineapple juices treated with immobilized pure pectinase showed greater clarity. Following treatment with pure pectinase, the fruit juice's 1, 1-diphenyl-2-picrylhydrazyl and 2, 2'-azino-bis 3-ethylbenzothiazoline-6-sulfonate scavenging activities increased. Following treatment with pure pectinase, the amounts of total phenolics and total flavonoids increased.
Conclusion: The procedure is deemed cost-effective in the food industry because the strong affinity of fungal pectinase for pectin. The investigated pectinase supported its usage in the food industry by being able to clear orange, mango, and pineapple juices.
Keywords: Alginate; Antioxidant activity; Chitosan; Mango; Orange; Pectinase; Pineapple.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Research involving human participation and/or animals: Not applicable. Competing interests: The authors declare no competing interests.
Figures







References
-
- Abdel-Naby MA. Immobilization of Aspergillus niger NRC 107 xylanase and β-xylosidase, and properties of the immobilized enzymes. Appl Biochem Biotechnol. 1993;38:69–81. - PubMed
-
- Arasaratnam V, Mylvaganam K, Balasubramaniam K. Glucoamylase production by Aspergillus niger in solid state fermentation with paddy husk as support. J Food Sci Technol Mysore. 2001;38(4):334–8.
-
- Arora NK, Jitendra M, Vaibhav M. Microbial enzymes: roles and applications in industries. Berlin Heidelberg: Springer; 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

