European Guideline on Chronic Nausea and Vomiting-A UEG and ESNM Consensus for Clinical Management
- PMID: 39754724
- PMCID: PMC11999049
- DOI: 10.1002/ueg2.12711
European Guideline on Chronic Nausea and Vomiting-A UEG and ESNM Consensus for Clinical Management
Abstract
Introduction: Chronic nausea and vomiting are symptoms of a wide range of gastrointestinal and non-gastrointestinal conditions. Diagnosis can be challenging and requires a systematic and well-structured approach. If the initial investigation for structural, toxic and metabolic disorders is negative, digestive motility and gut-brain interaction disorders should be assessed. United European Gastroenterology (UEG) and the European Society for Neurogastroenterology and Motility (ESNM) identified the need for an updated, evidence-based clinical guideline for the management of chronic nausea and vomiting.
Methods: A multidisciplinary team of experts in the field, including European specialists and national societies, participated in the development of the guideline. Relevant questions were addressed through a literature review and statements were developed and voted on according to a Delphi process.
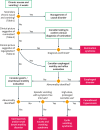
Results: Ninety-eight statements were identified and voted following the Delphi process. Overall agreement was high, although the grade of scientific evidence was low in many areas. Disagreement was more evident for some pharmacological treatment options. A diagnostic algorithm was developed, focussing on the differentiating features between gastrointestinal motility and gut-brain interaction disorders with predominant nausea and vomiting.
Conclusion: These guidelines provide an evidence-based framework for the evaluation and treatment of patients with chronic nausea and vomiting.
Keywords: Delphi; guidelines; nausea; vomiting.
© 2025 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
C.M. reports receipt of research support from Alfasigma. D.K. acknowledges receipt of grants and research support from UEG (guideline for faecal incontinence), MLDS (Dutch Foundation for Gastroenterology), Rome Foundation, ZonMw (Dutch governmental), and Horizon 2020 (EU). In addition, D.K. has received support from Allergan, Grunenthal, and Will Pharma. D.K. has participated in a company‐sponsored speaker’s bureau for Dr. Falk, with payments directed to the host institution. J.S. declares receiving grants and research support from Bayer and Laboratorios Salvat SA. Additionally, J.S. reports receiving advisory, honoraria, or consultant fees from Norgine and Reckitt Benckiser. J.S. has participated in company‐sponsored speaker’s bureaus for Norgine, Cassen Recordati, Bayer, and Grünenthal. M.C. discloses being a co‐investigator on a grant sponsored by Sanofi, and serving as a consultant for Sanofi, Mayoly, RB, and Arena. N.R. reports owning intellectual property in a patent family related to pressure‐flow analytics. R.D.G. reports having received grants and research support, as well as advisory, honoraria, or consultant fees from Kyowa Kirin International and Takeda. All other authors have declared no conflicts of interest in relation to this study.
Figures
References
-
- Fitch K., Bernstein S. J., Aguilar M. D., et al., RAND/UCLA Appropriateness Method User's Manual (Santa Monica, CA: RAND corporation, 2000).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

