Delayed atorvastatin delivery promotes recovery after experimental spinal cord injury
- PMID: 39755500
- PMCID: PMC12014417
- DOI: 10.1016/j.neurot.2024.e00517
Delayed atorvastatin delivery promotes recovery after experimental spinal cord injury
Abstract
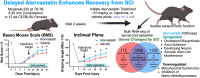
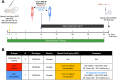
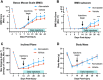
Spinal cord injury (SCI) significantly alters gene expression, potentially impeding functional recovery. This study investigated the effects of atorvastatin, a widely prescribed cholesterol-lowering drug, on gene expression and functional recovery in a chronic murine SCI model. Female C57BL/6J mice underwent moderate 0.25 mm lateral compression SCI and received daily atorvastatin (10 mg/kg) or vehicle-only injections from two weeks post-injury for four weeks. Sensorimotor functions were assessed using the Basso Mouse Scale (BMS), its subscore, and the inclined plane test. RNA sequencing of spinal cord tissues identified robust transcriptomic changes from SCI and a smaller subset from atorvastatin treatment. Atorvastatin enhanced sensorimotor recovery within two weeks of treatment initiation, with effects persisting to the experimental endpoint. Pathway analysis showed atorvastatin enriched neural regeneration processes including Fatty Acid Transport, Axon Guidance, and the Endocannabinoid Developing Neuron Pathway; improved mitochondrial function via increased TCA Cycle II and reduced Mitochondrial Dysfunction; and decreased Inhibition of Matrix Metalloproteases. Key gene drivers included Fabp7, Unc5c, Rest, and Klf4. Together, these results indicate atorvastatin's potential in chronic SCI recovery, especially where already indicated for cardiovascular protection.
Keywords: Chronic spinal cord injury; Locomotor recovery; Pathway analysis; RNA sequencing; Spinal cord transcriptomics.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Gu Q. US Department of Health and Human Services, Centers for Disease Control and; 2014. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. - PubMed
-
- Sillesen H., Amarenco P., Hennerici M.G., Callahan A., Goldstein L.B., Zivin J., et al. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–3302. - PubMed
-
- Sever P.S., Dahlöf B., Poulter N.R., Wedel H., Beevers G., Caulfield M., et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. - PubMed
-
- Collins R., Reith C., Emberson J., Armitage J., Baigent C., Blackwell L., et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. - PubMed
-
- Heidenreich P.A., Trogdon J.G., Khavjou O.A., Butler J., Dracup K., Ezekowitz M.D., et al. Forecasting the future of cardiovascular disease in the United States. Circulation. 2011;123:933–944. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

