D-ribose-5-phosphate inactivates YAP and functions as a metabolic checkpoint
- PMID: 39755622
- PMCID: PMC11700446
- DOI: 10.1186/s13045-024-01655-1
D-ribose-5-phosphate inactivates YAP and functions as a metabolic checkpoint
Abstract
Background: Targeting glucose uptake by glucose transporter (GLUT) inhibitors is a therapeutic opportunity, but efforts on GLUT inhibitors have not been successful in the clinic and the underlying mechanism remains unclear. We aim to identify the key metabolic changes responsible for cancer cell survival from glucose limitation and elucidate its mechanism.
Methods: The level of phosphorylated YAP was analyzed with Western blotting and Phos-tag immunoblotting. Glucose limitation-induced metabolic changes were analyzed using targeted metabolomics (600MRM). The anti-cancer role of metabolite was examined using colony formation assay and APCmin/+ mice. Co-immunoprecipitation, LS-MS, qRT-PCR, and immunofluorescence were performed to explore the underlying mechanisms.
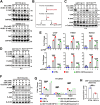
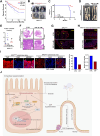
Results: We found that D-Ribose-5-phosphate (D5P), a product of the pentose phosphate pathway connecting glucose metabolism and nucleotide metabolism, functions as a metabolic checkpoint to activate YAP under glucose limitation to promote cancer cell survival. Mechanistically, in glucose-deprived cancer cells, D5P is decreased, which facilitates the interaction between MYH9 and LATS1, resulting in MYH9-mediated LATS1 aggregation, degradation, and further YAP activation. Interestingly, activated YAP further promotes purine nucleoside phosphorylase (PNP)-mediated breakdown of purine nucleoside to restore D5P in a feedback manner. Importantly, D5P synergistically enhances the tumor-suppressive effect of GLUT inhibitors and inhibits cancer progression in mice.
Conclusions: Our study identifies D5P as a metabolic checkpoint linking glucose limitation stress and YAP activation, indicating that D5P may be a potential anti-cancer metabolite by enhancing glucose limitation sensitivity.
Keywords: D-ribose-5-phosphate; Glucose deprivation; LATS1; Metabolic stress; Purine nucleoside phosphorylase; YAP.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Institutional Animal Care and Use Committee of the Zhengzhou University. All animal experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011) and according to the institutional ethical guidelines for animal experiments. Competing interests: The authors declare no competing interests.
Figures





References
-
- Chen X, Zhao Y, He C, Gao G, Li J, Qiu L, et al. Identification of a novel GLUT1 inhibitor with in vitro and in vivo anti-tumor activity. Int J Biol Macromol. 2022;216:768–78. - PubMed
-
- Katt WP, Lukey MJ, Cerione RA. Starving the devourer: cutting cancer off from its favorite foods. Cell Chem Biol. 2019;26(9):1197–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

