From ex vivo to in vivo chimeric antigen T cells manufacturing: new horizons for CAR T-cell based therapy
- PMID: 39755643
- PMCID: PMC11700462
- DOI: 10.1186/s12967-024-06052-3
From ex vivo to in vivo chimeric antigen T cells manufacturing: new horizons for CAR T-cell based therapy
Abstract
In the past decades, Chimeric Antigen Receptor (CAR)-T cell therapy has achieved remarkable success, leading to the approval of six therapeutic products for haematological malignancies. Recently, the therapeutic potential of this therapy has also been demonstrated in non-tumoral diseases. Currently, the manufacturing process to produce clinical-grade CAR-T cells is complex, time-consuming, and highly expensive. It involves multiple steps, including the collection of T cells from patients or healthy donors, in vitro engineering and expansion, and finally reinfusion into patients. Therefore, despite the impressive clinical outcomes, ex vivo manufacturing process makes CAR-T cells out of reach for many cancer patients. Direct in vivo engineering of T cells could be a more rapid solution able to circumvent both the complexity and the costs associated with ex vivo manufactured CAR-T cells. This novel approach allows to completely eliminate ex vivo cell manipulation and expansion while producing therapeutic cell populations directly in vivo. To date, several studies have demonstrated the feasibility of in vivo T cell reprogramming, by employing injectable viral- or nanocarrier-based delivery platforms in tumour animal models. Additionally, in vivo production of CAR-T cells might reduce the incidence, or at least the severity, of systemic toxicities frequently occurring with ex vivo produced CAR-T cells, such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. In this review, we highlight the challenges associated with the current ex vivo manufacturing protocols and review the latest progresses in the emerging field of in vivo CAR-T therapy, by comparing the various platforms so far investigated. Moreover, we offer an overview of the advantages deriving from in vivo reprogramming of other immune cell types, such as Natural Killer and macrophages, with CAR constructs.
Keywords: CAR T cell; Manufacturing; T cell engineering.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors consent to publication. Competing interests: The authors declare no competing interests.
Figures
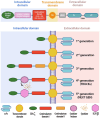


References
-
- Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc “spacer” domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17(10):1206–13. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

