Therapeutic reduction of neurocan in murine intracerebral hemorrhage lesions promotes oligodendrogenesis and functional recovery
- PMID: 39755654
- PMCID: PMC11699774
- DOI: 10.1186/s12974-024-03331-0
Therapeutic reduction of neurocan in murine intracerebral hemorrhage lesions promotes oligodendrogenesis and functional recovery
Abstract
Background: Intracerebral hemorrhage (ICH) causes prominent deposition of extracellular matrix molecules, particularly the chondroitin sulphate proteoglycan (CSPG) member neurocan. In tissue culture, neurocan impedes the properties of oligodendrocytes. Whether therapeutic reduction of neurocan promotes oligodendrogenesis and functional recovery in ICH is unknown.
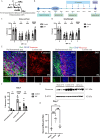
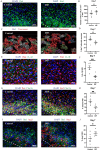
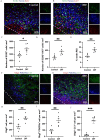
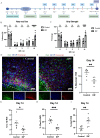
Methods: Mice were retro-orbitally injected with adeno-associated virus (AAV-CRISPR/Cas9) to reduce neurocan deposition after ICH induction by collagenase. Other groups of ICH mice were treated with vehicle or a drug that reduces CSPG synthesis, 4-4-difluoro-N-acetylglucosamine (difluorosamine). Rota-rod and grip strength behavioral tests were conducted over 7 or 14 days. Brain tissues were investigated for expression of neurocan by immunofluorescence microscopy and western blot analysis. Brain cryosections were also stained for microglia/macrophage phenotype, oligodendrocyte lineage cells and neuroblasts by immunofluorescence microscopy. Tissue structural changes were assessed using brain magnetic resonance imaging (MRI).
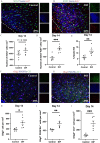
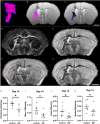
Results: The adeno-associated virus (AAV)-reduction of neurocan increased oligodendrocyte numbers and functional recovery in ICH. The small molecule inhibitor of CSPG synthesis, difluorosamine, lowered neurocan levels in lesions and elevated numbers of oligodendrocyte precursor cells, mature oligodendrocytes, and SOX2+ nestin+ neuroblasts in the perihematomal area. Difluorosamine shifted the degeneration-associated functional state of microglia/macrophages in ICH towards a regulatory phenotype. MRI analyses showed better fiber tract integrity in the penumbra of difluorosamine mice. These beneficial difluorosamine results were achieved with delayed (2 or 3 days) treatment after ICH.
Conclusion: Reducing neurocan deposition following ICH injury is a therapeutic approach to promote histological and behavioral recovery from the devastating stroke.
Keywords: Chondroitin sulphate proteoglycans; Extracellular matrix; Functional recovery; Intracerebral hemorrhage; Neurocan; Oligodendrogenesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed with ethics approval (protocol number AC21-0073) from the Animal Care Committee at the University of Calgary under regulations of the Canadian Council of Animal Care. Consent for publication: Not applicable. Competing interests: PZ, CCL and VWY are co-inventors in a US Provisional Patent application (US 63/720,938) titled: Fluorinated glucosamine analogs to reduce injury and promote recovery in neurological disorders.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

