Ultrasensitive detection and quantification of bovine Deltapapillomavirus in the semen of healthy horses
- PMID: 39755719
- PMCID: PMC11700219
- DOI: 10.1038/s41598-024-81682-7
Ultrasensitive detection and quantification of bovine Deltapapillomavirus in the semen of healthy horses
Abstract
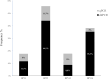
BPV1, BPV2, BPV13, and BPV14 are all genotypes of bovine delta papillomaviruses (δPV), of which the first three cause infections in horses and are associated with equine sarcoids. However, BPV14 infection has never been reported in equine species. In this study, we examined 58 fresh and thawed commercial semen samples from healthy stallions. In 34 (58.6%), bovine δPV DNA was detected and quantified using droplet digital polymerase chain reaction (ddPCR). Real time quantitative PCR (qPCR) was able to identify bovine δPV DNA in 5 samples (8.6%). Of the BPV-infected semen samples, 15 were positive for BPV2 (~ 44.1%) on ddPCR and 4 (~ 11.7%) on qPCR; 12 (~ 35.3%) for BPV14 on ddPCR and 1 (~ 3%) by qPCR; 4 (~ 11.7%) for BPV1 on ddPCR, whereas qPCR failed to reveal this infection; 3 (~ 8.8%) for BPV13 on ddPCR; and BPV13 infection was not detected by qPCR. Our study showed for the first time that BPV14 is an additional infectious agent potentially responsible for infection in horses, as its transcripts were detected and quantified in some semen samples. Large-scale BPV14 screening is necessary to provide substantial data on the molecular epidemiology for a better understanding of the geographical divergence of BPV14 prevalence in different areas and how widespread BPV14 is among equids.
Keywords: Bovine papillomavirus; Commercial stallion semen; Droplet digital PCR (ddPCR); Healthy stallions; Real time quantitative PCR (qPCR); Semen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Semen samples were commercially purchased. Some additional samples were collected at the Didactic Veterinary University Hospital (DVUH) of Naples. All animal studies were approved by the Institutional Animal Care and Use Committee (Protocol PG/2024/0023599, Naples University Federico II). Permission to collect samples was obtained from the animals’ owners.
Figures

References
-
- International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 90, Human Papillomaviruses, WHO, Lyon, France, 2007.
-
- PaVE: The papillomavirus episteme available from http://pave.niaid.nih.gov (accessed on 31 August 2024).
-
- Carvalho, T., Pinto, C. & Peleteiro, M. C. Urinary bladder lesions in bovine enzootic haematuria. J. Comp. Pathol.134, 336–346. 10.1016/j.jcpa.2006.01.001 (2006). - PubMed
-
- Wosiaki, S. R., Claus, M. P., Alfieri, A. F. & Alfieri, A. A. Bovine papillomavirus type 2 detection in the urinary bladder of cattle with chronic enzootic haematuria. Mem. Inst. Oswaldo Cruz.101, 635–638. 10.1590/s0074-02762006000600009 (2006). - PubMed
-
- Roperto, S. et al. A review of bovine urothelial tumours and tumour-like lesions of the urinary bladder. J. Comp. Pathol.142, 95–108. 10.1016/j.jcpa.2009.08.156 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

