ADAMDEC1 promotes the malignant progression of cholangiocarcinoma by regulating NF-κB signaling pathway
- PMID: 39755752
- PMCID: PMC11700112
- DOI: 10.1038/s41598-025-85241-6
ADAMDEC1 promotes the malignant progression of cholangiocarcinoma by regulating NF-κB signaling pathway
Abstract
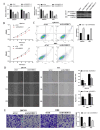
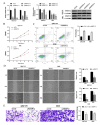
Cholangiocarcinoma (CCA), a highly aggressive form of cancer, is known for its high mortality rate. A Disintegrin and Metalloprotease Domain-like Protein Decysin-1 (ADAMDEC1) can promote the development and metastasis in various tumors by degrading the extracellular matrix. However, its regulatory mechanism in CCA remains unclear. Public databases and clinical tissue samples were used to evaluate whether ADAMDEC1 expression was correlated with the prognosis of CCA. We investigated the expression of ADAMDEC1-related regulatory genes and proteins in CCA and assessed the biological behaviors of CCA cells in vitro through functional experiments. Meanwhile, the interacting proteins of ADAMDEC1 and its involvement in the nuclear factor-kappa B (NF-κB) signaling pathway were screened and verified through bioinformatics analysis. The tumorigenicity of CCA was also assessed in a xenograft nude mouse model. Our results showed that ADAMDEC1 was highly expressed in tumor tissues from CCA patients and was positively correlated with poor prognosis. Interference cell lines targeting ADAMDEC1 in CCA cells were successfully constructed. Knockdown of ADAMDEC1 or MMP12 both affected the biological behaviors of CCA cells, and ADAMDEC1 silencing inhibited tumorigenicity and tumor growth of CCA in vivo. Moreover, ADAMDEC1 interacted with MMP12, modulating its expression and promoting the activation of the NF-κB signaling pathway. Our study uncovered the expression patterns and functional roles of ADAMDEC1 in CCA cells and tissues, highlighting its connection to the NF-κB pathway and MMP12 in the development of CCA. Therefore, ADAMDEC1 may serve as a potential therapeutic target for CCA.
Keywords: ADAMDEC1; CCA; MMP12; NF-κB; Tumorigenesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

