Cyclin E1/CDK2 activation defines a key vulnerability to WEE1 kinase inhibition in gynecological cancers
- PMID: 39755818
- PMCID: PMC11700143
- DOI: 10.1038/s41698-024-00787-4
Cyclin E1/CDK2 activation defines a key vulnerability to WEE1 kinase inhibition in gynecological cancers
Abstract
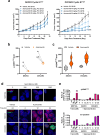
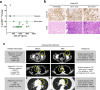
Upregulation of Cyclin E1 and subsequent activation of CDK2 accelerates cell cycle progression from G1 to S phase and is a common oncogenic driver in gynecological malignancies. WEE1 kinase counteracts the effects of Cyclin E1/CDK2 activation by regulating multiple cell cycle checkpoints. Here we characterized the relationship between Cyclin E1/CDK2 activation and sensitivity to the selective WEE1 inhibitor azenosertib. We found that ovarian cancer cell lines with high levels of endogenous Cyclin E1 expression or forced overexpression were exquisitely sensitive to azenosertib and these results extended to in vivo models of ovarian and uterine serous carcinoma. Models with high Cyclin E1 expression showed higher baseline levels of replication stress and enhanced cellular responses to azenosertib treatment. We found azenosertib synergized with different classes of chemotherapy and described distinct underlying mechanisms. Finally, we provided early evidence from an ongoing phase I study demonstrating the clinical activity of monotherapy azenosertib in patients with Cyclin E1/CDK2-activated ovarian and uterine serous carcinomas.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D. Kim is an employee and shareholder of Zentalis Pharmaceuticals. H. Chung is an employee and shareholder of Zentalis Pharmaceuticals. W. Liu is an employee and shareholder of Zentalis Pharmaceuticals. S. Kim is an employee and shareholder of Zentalis Pharmaceuticals. X. Guo is an employee and shareholder of Zentalis Pharmaceuticals. N. Jameson is an employee and shareholder of Zentalis Pharmaceuticals. P.R. de Jong is a former employee of Zentalis Pharmaceuticals. S. Yea is a former employee and shareholder of Zentalis Pharmaceuticals. L. Harford is a former employee and shareholder of Zentalis Pharmaceuticals. J. Li is a former employee of Zentalis Pharmaceuticals. D. Kim is an employee and shareholder of Zentalis Pharmaceuticals. K. Fischer is an employee and shareholder of Zentalis Pharmaceuticals. A. Samatar is a former employee of Zentalis Pharmaceuticals and shareholder in Zentalis Pharmaceuticals. A. Jubb is a former employee and shareholder of Zentalis Pharmaceuticals. K. Bunker is a former employee of Zentalis Pharmaceuticals and shareholder in Zentalis Pharmaceuticals. K. Blackwell is a former employee and shareholder of Zentalis Pharmaceuticals. F. Simpkins serves on scientific advisory boards for AstraZeneca, GSK and Zentalis Pharmaceuticals; has received institutional research funding from AstraZeneca, Repare Therapeutics, Instill Bio and Sierra Oncology. F. Meric-Bernstam is consultant for. AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, Daiichi Sankyo Co. Ltd., Calibr (a division of Scripps Research), DebioPharm, Ecor1 Capital, eFFECTOR Therapeutics, Exelixis, F. Hoffman-La Roche Ltd., GT Apeiron, Genentech Inc., Harbinger Health, IBM Watson, Incyte, Infinity Pharmaceuticals, Jackson Laboratory, Jazz Pharmaceuticals, Kolon Life Science, LegoChem Bio, Lengo Therapeutics, Loxo Oncology, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Protai Bio Ltd, Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks; Advisory Committee for Black Diamond, Biovica, Eisai, FogPharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, Theratechnologies, Zentalis; Received sponsored Research (to the institution) from Jazz Pharmaceuticals, Zymeworks, Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., Taiho Pharmaceutical Co.; Honoraria for Dava Oncology; receiving travel related funding and reimbursement from European Organization for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), Cholangiocarcinoma Foundation, Dava Oncology. G.B. Mills is scientific advisory board/Consultant for Amphista, Astex, AstraZeneca, BlueDot, Chrysallis Biotechnology, Ellipses Pharma, GSK, ImmunoMET, Infinity, Ionis, Leapfrog Bio, Lilly, Medacorp, Nanostring, Nuvectis, PDX Pharmaceuticals, Qureator, Roche, Signalchem Lifesciences, Tarveda, Turbine, Zentalis Pharmaceuticals; Stock/Options/Financial: Bluedot, Catena Pharmaceuticals, ImmunoMet, Nuvectis, SignalChem, Tarveda, Turbine; Licensed Technology: HRD assay to Myriad Genetics, DSP patents with Nanostring; Sponsored research: AstraZeneca. O. Harismendy is an employee and shareholder of Zentalis Pharmaceuticals. J. Ma is an employee and shareholder of Zentalis Pharmaceuticals. M.R. Lackner is an employee and shareholder of Zentalis Pharmaceuticals. No disclosures were reported by the other authors.
Figures




References
-
- CDC-Ovarian Cancer Statistics [Internet]. [cited 2023 Oct 30]. Available from: https://www.cdc.gov/cancer/ovarian/statistics/index.htm
-
- SEER Cancer Stat Facts: ovarian cancer [Internet]. [cited 2023 Oct 30]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html
LinkOut - more resources
Full Text Sources

