Vanzacaftor-tezacaftor-deutivacaftor for children aged 6-11 years with cystic fibrosis (RIDGELINE Trial VX21-121-105): an analysis from a single-arm, phase 3 trial
- PMID: 39756425
- PMCID: PMC12126198
- DOI: 10.1016/S2213-2600(24)00407-7
Vanzacaftor-tezacaftor-deutivacaftor for children aged 6-11 years with cystic fibrosis (RIDGELINE Trial VX21-121-105): an analysis from a single-arm, phase 3 trial
Erratum in
-
Correction to Lancet Respir Med 2025; 13: 244-55.Lancet Respir Med. 2025 Mar;13(3):e19. doi: 10.1016/S2213-2600(25)00010-4. Lancet Respir Med. 2025. PMID: 40049937 No abstract available.
Abstract
Background: In phase 2 trials in people with cystic fibrosis aged 18 years and older, vanzacaftor-tezacaftor-deutivacaftor has been shown to be a safe and effective, once-daily cystic fibrosis transmembrane conductance regulator (CFTR) modulator. Restoring normal CFTR function early in life has the potential to prevent manifestations of cystic fibrosis. We aimed to evaluate the safety, tolerability, efficacy, and pharmacokinetics of vanzacaftor-tezacaftor-deutivacaftor in children with cystic fibrosis aged 6-11 years.
Methods: In this multicentre, single-arm, phase 3 trial (RIDGELINE Trial VX21-121-105), participants were enrolled across 33 clinical sites that care for children with cystic fibrosis in eight countries (Australia, France, Germany, Netherlands, Sweden, Switzerland, the UK, and the USA). Eligible participants were aged 6-11 years with at least one elexacaftor-tezacaftor-ivacaftor-responsive CFTR variant, FEV1 % predicted of 60% or higher, and stable cystic fibrosis as determined by investigators. Before study treatment, participants were either on stable elexacaftor-tezacaftor-ivacaftor for at least 28 days before screening or received the combination for a 4-week run-in period. Participants then received vanzacaftor-tezacaftor-deutivacaftor (<40 kg bodyweight: vanzacaftor 12 mg, tezacaftor 60 mg, and deutivacaftor 150 mg orally as three fixed-dose combination tablets once daily; ≥40 kg bodyweight: vanzacaftor 20 mg, tezacaftor 100 mg, and deutivacaftor 250 mg orally as two fixed-dose combination tablets once daily (manufactured by Patheon Pharmaceuticals, Cincinnati, OH, USA) from day 1 for 24 weeks. The primary endpoint was safety and tolerability, as measured by adverse events, vital signs, clinical laboratory values, electrocardiograms, and pulse oximetry. Endpoints were analysed in all participants who received at least one dose of vanzacaftor-tezacaftor-deutivacaftor. This trial is registered with ClinicalTrials.gov, NCT05422222, and evaluation of the 6-11-year-old cohort is complete.
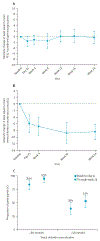
Findings: Between Feb 6 and May 18, 2023, 83 children were screened, of whom five were not eligible, and 78 children aged 6-11 years received at least one dose of vanzacaftor-tezacaftor-deutivacaftor. Median age was 9·3 years (IQR 7·6-10·4), 34 (44%) of 78 participants were female, 44 (56%) were male, 71 (91%) were White, one (1%) was Black or African American, and one (1%) was of multiple races. The analysis for these data was completed on Dec 15, 2023. Median exposure of participants to vanzacaftor-tezacaftor-deutivacaftor was 168 days (IQR 166-170). 75 (96%) of 78 participants had adverse events, all of which were mild or moderate; the most common events were generally consistent with cystic fibrosis manifestations, including, cough (36 [46%]), pyrexia (16 [21%]), headache (14 [18%]), infective pulmonary exacerbation of cystic fibrosis (13 [17%]), and oropharyngeal pain (13 [17%]). Serious adverse events occurred in six (8%) participants (two had infective pulmonary exacerbation, one of whom also had failure to thrive; one participant each had adenovirus infection, constipation, pulmonary function test decreased, and cough), and one (1%) participant discontinued due to adverse events of cough and fatigue that were considered possibly related to study drug.
Interpretation: Vanzacaftor-tezacaftor-deutivacaftor was safe and well tolerated and maintained FEV1 % predicted from elexacaftor-tezacaftor-ivacaftor baseline with further improved CFTR function. Improvements in CFTR function compared with baseline elexacaftor-tezacaftor-ivacaftor values demonstrate the potential opportunity to restore normal physiology early and prevent development or progression of cystic fibrosis. Nearly all participants had sweat chloride below the diagnostic threshold for cytstic fibrosis (<60 mmol/L) and more than half had normal levels (<30 mmol/L). Additional long-term data in children with cystic fibrosis are being collected in an open-label extension study to demonstrate clinical benefits and safety. These findings will inform health-care providers and people with cystic fibrosis regarding the benefits of early initiation of CFTR modulators.
Funding: Vertex Pharmaceuticals.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests DBS, JZ, Y-CC, VR-R, and PRS are employees of Vertex Pharmaceuticals and might own stock or have stock options in that company. JEH reports grants from Cystic Fibrosis Foundation and the US National Institutes of Health (NIH) and support to travel or attend meetings from Vertex Pharmaceuticals, Cystic Fibrosis Foundation, and Cystic Fibrosis Canada. ASK reports grants from Vertex Pharmaceuticals and Cystic Fibrosis Foundation and support to attend meetings from Vertex Pharmaceuticals. JEP reports grants to their institution from Cystic Fibrosis Foundation Therapeutics Development Center; honorarium for participation in Grant Review Committee from the Cystic Fibrosis Foundation Clinical Review Committee; and reduced registration fee from the North American Cystic Fibrosis Conference and American Thoracic Society Conference. RJ reports their institution was engaged as central over-readers for the multiple breath washout data collected in this Vertex trial. LPT reports advisory board participation and honoraria from Vertex Pharmaceuticals; funding to her institution to support educational materials for health-care professionals from Vertex Pharmaceuticals, AstraZeneca, and GSK; and participation on a Cystic Fibrosis Trust research oversight advisory board. BR reports grants from the NIH and the Cystic Fibrosis Foundation; consulting fees from Vertex Pharmaceuticals, Cystetic Medicines, and Sionna Therapeutics; and participation on a data safety monitoring or advisory board for the NIH. MAM reports grants from the German Research Foundation, German Ministry for Education and Research, German Innovation Fund, Vertex Pharmaceuticals, and Boehringer Ingelheim; consulting fees from AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Splisense, and Vertex Pharmaceuticals; speaker fees from Vertex Pharmaceuticals; travel support from Boehringer Ingelheim and Vertex Pharmaceuticals; participation on a data safety monitoring board or advisory board for AbbVie, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Pari, and Vertex Pharmaceuticals; and is a fellow of the European Respiratory Society. JLT-C reports grants and consulting fees from Vertex Pharmaceuticals and 4D Molecular Therapeutics; grant from Eloxx; participation on a data safety monitoring board or advisory board for AbbVie; serving as the adult patient care representative for the Cystic Fibrosis Board of Trustees; serving on the Cystic Fibrosis Foundation Clinical Research Executive Committee; serving as immediate past chair of the Cystic Fibrosis Therapeutic Development Network's Sexual Health, Reproduction and Gender Research Working Group; serving as co-chair of the Health Equity Team Science Awards study section and on the Racial Justice Working Group; serving on the scientific advisory board for Emily's Entourage; previously serving on the American Thoracic Society Respiratory Health Awards and Scientific Grant Review Committees; serving as Chair-elect of the International Conference Committee; serving as Associate Editor for the Journal of Cystic Fibrosis; serving as a member of the International Advisory Board for The Lancet Respiratory Medicine; and serving on the Clinical Trials Review study section for the NIH. EFM reports grants and speaker fees from Vertex Pharmaceuticals; travel support from Menarini; and participation on a data safety monitoring board or advisory board for CF STORM Clinical Trial, Vertex Pharmaceuticals, Janssen, AbbVie, Insmed, and Enterprise Therapeutics. ET reports grants from St Michael's Hospital and consulting fees, speaker fees, and travel support from Vertex Pharmaceuticals. GD reports institutional fees, meeting support, and advisory board participation from Vertex Pharmaceuticals and speaker honoraria for educational events from Vertex Pharmaceuticals and Chiesi. PR and PT declare no competing interests.
Figures
References
-
- Cystic Fibrosis Foundation. 2022 annual data report. Bethesda, MD: Cystic Fibrosis Foundation, 2023.
-
- Zolin A, Orenti A, Adarmoli A, et al. ECFSPR annual report 2021. Karup: European Cystic Fibrosis Society, 2023.
-
- Mall MA, Burgel PR, Castellani C, Davies JC, Salathe M, Taylor-Cousar JL. Cystic fibrosis. Nat Rev Dis Primers 2024; 10: 53. - PubMed
-
- Wilschanski M, Peckham D. Nutritional and metabolic management for cystic fibrosis in a post-cystic fibrosis transmembrane conductance modulator era. Curr Opin Pulm Med 2022; 28: 577–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

