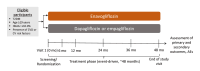
Study Design and Protocol for a Randomized Controlled Trial of Enavogliflozin to Evaluate Cardiorenal Outcomes in Type 2 Diabetes (ENVELOP)
- PMID: 39756817
- PMCID: PMC11960196
- DOI: 10.4093/dmj.2024.0238
Study Design and Protocol for a Randomized Controlled Trial of Enavogliflozin to Evaluate Cardiorenal Outcomes in Type 2 Diabetes (ENVELOP)
Abstract
Background: The novel sodium-glucose cotransporter-2 (SGLT2) inhibitor enavogliflozin effectively lowers glycosylated hemoglobin levels and body weights without the increased risk of serious adverse events; however, the long-term clinical benefits of enavogliflozin in terms of cardiovascular and renal outcomes have not been investigated.
Methods: This study is an investigator-initiated, multicenter, randomized, pragmatic, open-label, active-controlled, non-inferiority trial. Eligible participants are adults (aged ≥19 years) with type 2 diabetes mellitus (T2DM) who have a history of, or are at risk of, cardiovascular disease. A total of 2,862 participants will be randomly assigned to receive either enavogliflozin or other SGLT2 inhibitors with proven cardiorenal benefits, such as dapagliflozin or empagliflozin. The primary endpoint is the time to the first occurrence of a composite of major adverse cardiovascular or renal events (Clinical Research Information Service registration number: KCT0009243).
Conclusion: This trial will determine whether enavogliflozin is non-inferior to dapagliflozin or empagliflozin in terms of cardiorenal outcomes in patients with T2DM and cardiovascular risk factors. This study will elucidate the role of enavogliflozin in preventing vascular complications in patients with T2DM.
Keywords: Cardiovascular diseases; Diabetes mellitus, type 2; Enavogliflozin; Kidney diseases; Randomized controlled trial; Sodium- glucose transporter 2 inhibitors.
Conflict of interest statement
In-Kyung Jeong has been an honorary editor of the
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas 2021. 10th ed. Brussels: IDF; 2021. - PubMed
-
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. - PubMed
-
- Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. - PubMed
-
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical