Celecoxib is the only nonsteroidal anti-inflammatory drug to inhibit bone progression in spondyloarthritis
- PMID: 39757202
- PMCID: PMC11955732
- DOI: 10.5483/BMBRep.2024-0062
Celecoxib is the only nonsteroidal anti-inflammatory drug to inhibit bone progression in spondyloarthritis
Erratum in
-
Erratum to: Celecoxib is the only nonsteroidal anti-inflammatory drug to inhibit bone progression in spondyloarthritis.BMB Rep. 2025 Apr;58(4):190. BMB Rep. 2025. PMID: 40296671 Free PMC article.
Abstract
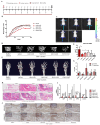
Spondyloarthritis (SpA) is a chronic inflammatory disease that leads to ankylosis of the axial skeleton. Celecoxib (cyclooxygenase-2 inhibitor, COX-2i) inhibited radiographic progression in a clinical study of SpA, but in the following study, diclofenac (COX-2 non-selective) failed to show that inhibition. Our study aimed to investigate whether nonsteroidal anti-inflammatory drugs (NSAIDs) inhibited bone progression in SpA, and whether celecoxib had a unique function (independent of the COX-inhibitor), compared with the other NSAIDs. We investigated the efficacy of various NSAIDs in curdlan-injected SKG mice (SKGc), an animal model of SpA, analyzed by bone micro-CT and immunohistochemistry. We also tested the effect of NSAIDs on osteoblast (OB) differentiation and bone mineralization in primary bone-derived cells (BdCs) from mice, and in ankylosing spondylitis (AS) patients and human osteosarcoma cell line (SaOS2). Celecoxib significantly inhibited clinical arthritis and bone progression in the joints of SKGc, but not etoricoxib (another COX-2i), nor naproxen (COX-2 nonselective). Both DM-celecoxib, not inhibiting COX-2, and celecoxib, inhibited OB differentiation and bone mineralization in the BdCs of mice and AS patients, and in SaOS2, but etoricoxib or naproxen did not. The in silico study indicated that celecoxib and 2,5-dimethyl-celecoxib (DM-celecoxib) would bind to cadherin-11 (CDH11) with higher affinity than etoricoxib and naproxen. Celecoxib suppressed CDH11-mediated β-catenin signaling in the joints of SKGc, primary mice cells, and SaOS2 cells. Of the NSAIDs, only celecoxib inhibited bone progression in SKGc and OB differentiation and bone mineralization in the BdCs of mice and AS patients via CDH11/WNT signaling, independent of the COX-2 inhibition. [BMB Reports 2025; 58(3): 140-145].
Conflict of interest statement
The authors have no conflicting interests.
Figures



References
-
- Emond B, Ellis LA, Chakravarty SD, Ladouceur M, Lefebvre P. Real-world incidence of inflammatory bowel disease among patients with other chronic inflammatory diseases treated with interleukin-17a or phosphodiesterase 4 inhibitors. Curr Med Res Opin. 2019;35:1751–1759. doi: 10.1080/03007995.2019.1620713. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

