Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis
- PMID: 39757285
- PMCID: PMC11751354
- DOI: 10.1007/s40744-024-00736-4
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis
Erratum in
-
Correction: Real‑World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis.Rheumatol Ther. 2025 Dec;12(6):1193-1196. doi: 10.1007/s40744-025-00782-6. Rheumatol Ther. 2025. PMID: 40944846 Free PMC article. No abstract available.
Abstract
Introduction: This study sought to describe treatment patterns, persistence, and effectiveness of upadacitinib (UPA) alone and compared to other Janus kinase inhibitors (JAKis) or tumor necrosis factor inhibitors (TNFis) in patients with rheumatoid arthritis (RA).
Methods: This retrospective, non-interventional study used the OPAL dataset, derived from electronic medical records. Patients initiated UPA (N = 2624), other JAKis (baricitinib and tofacitinib [N = 925]), or TNFis (adalimumab, etanercept, certolizumab, golimumab, infliximab [N = 3540]) between May 2020 and March 2023. Median persistence (Kaplan-Meier) and effectiveness (Disease Activity Score 28-joint C-reactive protein, three variables [DAS28CRP{3}]) were evaluated for UPA-treated patients and in three propensity score-matched cohorts: UPA monotherapy versus combination therapy, UPA versus other JAKis, and UPA versus TNFis.
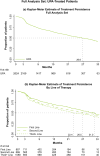
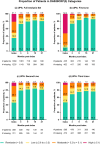
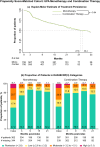
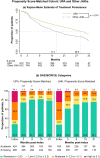
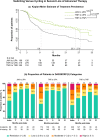
Results: In patients prescribed UPA, 41.3% were ≥ 65 years old, 33.8% were prescribed as first-line advanced therapy, and 27.2% were prescribed monotherapy. Persistence on UPA was 26.6 months (95% confidence intervals: 24.4, 29.9) and longest in earlier lines of therapy. The DAS28CRP(3) remission rate was 73% at 3 months, with improvements observed across lines of therapy. UPA monotherapy and combination therapy had similar persistence (27.8 [23.5, 33.4] versus 30.4 months [22.1, 35.3], p = 0.84) and effectiveness. UPA showed longer persistence than other JAKis (28.8 [25.6, 32.4] versus 17.2 months [14.9, 19.8], p < 0.001) and TNFis (26.6 [24.9, 30.8] versus 13.3 months [11.5, 14.5], p < 0.001). DAS28CRP(3) remission rates were greater at 3 months for UPA than other JAKis (75.0% versus 61.5%) and TNFis (72.7% versus 59.5%). In unmatched subgroups, compared to cycling between TNFis, switching to UPA from other JAKis or TNFis resulted in longer persistence (JAKi-to-UPA: 25.3 [16.1, not reached]; TNFi-to-UPA: 27.8 [23.2, 35.4]; TNFi-to-TNFi: 9.6 [8.4, 10.7]) and greater DAS28CRP(3) remission rates over 9 months.
Conclusions: Overall, the breadth and depth of data from this large real-world dataset continue to support a favorable clinical profile of UPA for the treatment of RA and may inform treatment choices in everyday clinical practice.
Keywords: Australia; Biologic disease-modifying antirheumatic drug (bDMARD); Janus kinase inhibitor (JAKi); Persistence; Real-world effectiveness; Remission; Rheumatoid arthritis (RA); Targeted synthetic disease-modifying antirheumatic drug (tsDMARD); Tumor necrosis factor inhibitor (TNFi); Upadacitinib (UPA).
Plain language summary
This study of patients treated in clinical practice looked at how a targeted medication, a Janus kinase inhibitor (JAKi) called upadacitinib, was used to treat rheumatoid arthritis, a disease that causes joint pain and damage. The researchers wanted to see how long patients continued treatment with upadacitinib, how effective it was at reducing symptoms, and how these outcomes compared to other JAKis (baricitinib and tofacitinib) and tumor necrosis factor inhibitors, a different type of advanced medication. Patients continued upadacitinib treatment for a median time of over 2 years. Those patients who had never previously used JAKis or tumor necrosis factor inhibitors continued upadacitinib treatment longer than those patients with previous experience. Patients treated with upadacitinib alone or in combination with less advanced medications like methotrexate continued treatment for a similar length of time. Compared to other JAKis and tumor necrosis factor inhibitors, patients prescribed upadacitinib stayed on their treatment the longest. Upadacitinib helped to lessen the symptoms of rheumatoid arthritis, whether patients took it alone or in combination with medications like methotrexate. After 3 months, more patients treated with upadacitinib experienced reduced symptoms than patients treated with other JAKis or tumor necrosis factor inhibitors. Additionally, patients who switched to upadacitinib after using other JAKis or tumor necrosis factor inhibitors reduced their symptoms and continued treatment for longer than those switching between tumor necrosis factor inhibitors. Overall, patients treated with upadacitinib continued treatment for longer and saw greater improvements in the symptoms of rheumatoid arthritis than patients prescribed other advanced medications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Financial arrangements of the authors with companies whose products may be related to the present manuscript are listed, as declared by the authors. Peter Youssef: has received honoraria for lectures and consulting from AbbVie, AstraZeneca, Eli Lilly, Celltrion, Pfizer, Sandoz, Janssen, and Novartis. Sabina Ciciriello and Talib Tahir: Nothing to disclose. Belinda Butcher: Acts as a statistical consultant to many pharmaceutical and device companies, including those who manufacture products for RA. Joanna Leadbetter: Acts as a statistical consultant to many pharmaceutical and device companies, including those who manufacture products for RA. Tegan Smith: Former employee of OPAL Rheumatology Ltd, current employee of Hunter Medical Research Institute, Newcastle, NSW, Australia. Catherine O’Sullivan: Former employee of OPAL Rheumatology Ltd. Miriam Calao: Employee of AbbVie Pty Ltd. Nicole Walsh: Employee of AbbVie Pty Ltd. and may hold stock or options. Geoffrey Littlejohn: has received honoraria for lectures, presentations, consulting, or support/sponsorship for scientific conferences from AbbVie, AstraZeneca, Gilead, and GSK. Ethical Approval: The activities of OPAL Rheumatology Ltd have received overarching ethics approval from the University of New South Wales Human Research Ethics Committee (HC17799), based on opt-out patient consent. This protocol was approved by the University of New South Wales Human Research Ethics Committee (HC230281). Inclusion of data in the OPAL dataset was based on opt-out consent, all data are de-identified.
Figures


References
-
- Youssef P, Ciciriello S, Tahir T, Smith T, O’Sullivan C, Leadbetter J et al. Persistence, effectiveness and treatment patterns of upadacitinib in over 2600 Australian rheumatoid arthritis patients: a retrospective analysis from the OPAL dataset - ACR meeting abstracts. Arthritis Rheumatol. Available from: https://acrabstracts.org/abstract/persistence-effectiveness-and-treatmen....
-
- Ciciriello S, Youssef P, Tahir T, Smith T, O’Sullivan C, Leadbetter J et al. Upadacitinib vs TNFi and other JAKi treatment outcomes in Australian rheumatoid arthritis patients: descriptive comparison of persistence and effectiveness using the OPAL dataset - ACR meeting abstracts. Arthritis Rheumatol; p.76. Available from: https://acrabstracts.org/abstract/upadacitinibvs-tnfi-and-other-jaki-tre....
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

