Nonenzymatic lysine D-lactylation induced by glyoxalase II substrate SLG dampens inflammatory immune responses
- PMID: 39757301
- PMCID: PMC11770101
- DOI: 10.1038/s41422-024-01060-w
Nonenzymatic lysine D-lactylation induced by glyoxalase II substrate SLG dampens inflammatory immune responses
Abstract
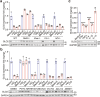
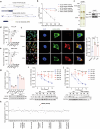
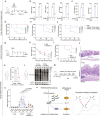
Immunometabolism is critical in the regulation of immunity and inflammation; however, the mechanism of preventing aberrant activation-induced immunopathology remains largely unclear. Here, we report that glyoxalase II (GLO2) in the glycolysis branching pathway is specifically downregulated by NF-κB signaling during innate immune activation via tristetraprolin (TTP)-mediated mRNA decay. As a result, its substrate S-D-lactoylglutathione (SLG) accumulates in the cytosol and directly induces D-lactyllysine modification of proteins. This nonenzymatic lactylation by SLG is greatly facilitated by a nearby cysteine residue, as it initially reacts with SLG to form a reversible S-lactylated thiol intermediate, followed by SN-transfer of the lactyl moiety to a proximal lysine. Lactylome profiling identifies 2255 lactylation sites mostly in cytosolic proteins of activated macrophages, and global protein structure analysis suggests that proximity to a cysteine residue determines the susceptibility of lysine to SLG-mediated D-lactylation. Furthermore, lactylation is preferentially enriched in proteins involved in immune activation and inflammatory pathways, and D-lactylation at lysine 310 (K310) of RelA attenuates inflammatory signaling and NF-κB transcriptional activity to restore immune homeostasis. Accordingly, TTP-binding site mutation or overexpression of GLO2 in vivo blocks this feedback lactylation in innate immune cells and promotes inflammation, whereas genetic deficiency or pharmacological inhibition of GLO2 restricts immune activation and attenuates inflammatory immunopathology both in vitro and in vivo. Importantly, dysregulation of the GLO2/SLG/D-lactylation regulatory axis is closely associated with human inflammatory phenotypes. Overall, our findings uncover an immunometabolic feedback loop of SLG-induced nonenzymatic D-lactylation and implicate GLO2 as a promising target for combating clinical inflammatory disorders.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Afonina, I. S., Zhong, Z., Karin, M. & Beyaert, R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat. Immunol.18, 861–869 (2017). - PubMed
-
- Raghuraman, S., Donkin, I., Versteyhe, S., Barres, R. & Simar, D. The emerging role of epigenetics in inflammation and immunometabolism. Trends Endocrinol. Metab.27, 782–795 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

